Sublethal Levels of Antibiotics Promote Bacterial Persistence in Epithelial Cells
- PMID: 32999821
- PMCID: PMC7509632
- DOI: 10.1002/advs.201900840
Sublethal Levels of Antibiotics Promote Bacterial Persistence in Epithelial Cells
Abstract
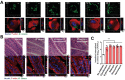
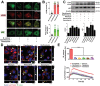
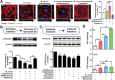
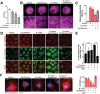
Antibiotic therapy and host cells frequently fail to eliminate invasive bacterial pathogens due to the emergence of antibiotic resistance, resulting in the relapse and recurrence of infections. Bacteria evolve various strategies to persist and survive in epithelial cells, a front-line barrier of host tissues counteracting invasion; however, it remains unclear how bacteria hijack cellular responses to promote cytoplasmic survival under antibiotic therapy. Here, it is demonstrated that extracellular bacteria show invasive behavior and survive in epithelial cells in both in vivo and in vitro models, to increase antibiotic tolerance. In turn, sublethal levels of antibiotics increase bacterial invasion through promoting the production of bacterial virulence factors. Furthermore, antibiotic treatments interrupt lysosomal acidification in autophagy due to the internalized bacteria, using Bacillus cereus and ciprofloxacin as a model. In addition, it is found that sublethal levels of ciprofloxacin cause mitochondrial dysfunction and reactive oxygen species (ROS) accumulation to impair lysosomal vascular tape ATPase (V-ATPase) to further promote bacterial persistence. Collectively, these results highlight the potential of host cells mediated antibiotic tolerance, which markedly compromises antibiotic efficacy and worsens the outcomes of infection.
Keywords: antibiotic; antibiotic tolerance; autophagy; bacteria; epithelial cells.
© 2020 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
