Histone deacetylase 4 deletion results in abnormal chondrocyte hypertrophy and premature ossification from collagen type 2α1‑expressing cells
- PMID: 33000215
- PMCID: PMC8369256
- DOI: 10.3892/mmr.2020.11465
Histone deacetylase 4 deletion results in abnormal chondrocyte hypertrophy and premature ossification from collagen type 2α1‑expressing cells
Abstract
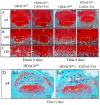
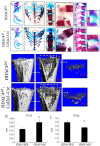
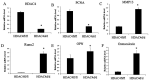
Histone deacetylase 4 (HDAC4) plays a vital role in chondrocyte hypertrophy and bone formation. To investigate the function of HDAC4 in postnatal skeletal development, the present study developed lineage‑specific HDAC4‑knockout mice [collagen type 2α1 (Col2α1)‑Cre, HDAC4d/d mice] by crossing transgenic mice expressing Cre recombinase. Thus, a specific ablation of HDAC4 was performed in Col2α1‑expressing mice cells. The knee joints of HDAC4fl/fl and Col2α1‑Cre, HDAC4d/d mice were analyzed at postnatal day (P)2‑P21 using an in vivo bromodeoxyuridine (BrdU) assay, and Safranin O, Von Kossa and whole‑body staining were used to evaluate the developmental growth plate, hypertrophic differentiation, mineralization and skeletal mineralization patterns. The trabecular bone was analyzed using microcomputed tomography. The expressions of BrdU, proliferating cell nuclear antigen (PCNA), matrix metalloproteinase (MMP)‑13, runt‑related transcription factor (Runx)‑2, osteoprotegerin (OPG), CD34, type X collagen (ColX), osteocalcin and Wnt5a were determined using immunohistochemistry, in situ hybridization (ISH) and reverse transcription‑quantitative (RT‑q)PCR. The results demonstrated that HDAC4‑null mice (HDAC4d/d mice) were severely runted; these mice had a shortened hypertrophic zone (histopathological evaluation), accelerated vascular invasion and articular mineralization (Von Kossa staining), elevated expressions of MMP‑13, Runx2, OPG and CD34 (RT‑qPCR and immunohistochemistry), downregulated expression of the proliferative marker BrdU and PCNA (immunohistochemistry), increased expression of ColX and decreased expression of Wnt5a (ISH). In conclusion, chondrocyte‑derived HDAC4 was responsible for regulating chondrocyte proliferation and differentiation as well as endochondral bone formation.
Figures



Similar articles
-
Conditional deletion of HDAC4 from collagen type 2α1-expressing cells increases angiogenesis in vivo.Mol Med. 2020 May 1;26(1):36. doi: 10.1186/s10020-020-00154-6. Mol Med. 2020. PMID: 32354322 Free PMC article.
-
MMP-13 is one of the critical mediators of the effect of HDAC4 deletion on the skeleton.Bone. 2016 Sep;90:142-51. doi: 10.1016/j.bone.2016.06.010. Epub 2016 Jun 16. Bone. 2016. PMID: 27320207 Free PMC article.
-
MicroRNA-1 regulates chondrocyte phenotype by repressing histone deacetylase 4 during growth plate development.FASEB J. 2014 Sep;28(9):3930-41. doi: 10.1096/fj.13-249318. Epub 2014 May 23. FASEB J. 2014. PMID: 24858276 Free PMC article.
-
Endochondral ossification signals in cartilage degradation during osteoarthritis progression in experimental mouse models.Mol Cells. 2008 Feb 29;25(1):1-6. Mol Cells. 2008. PMID: 18319608 Review.
-
Hdac-mediated control of endochondral and intramembranous ossification.Crit Rev Eukaryot Gene Expr. 2011;21(2):101-13. doi: 10.1615/critreveukargeneexpr.v21.i2.10. Crit Rev Eukaryot Gene Expr. 2011. PMID: 22077150 Free PMC article. Review.
Cited by
-
Regulation of FGF-2, FGF-18 and Transcription Factor Activity by Perlecan in the Maturational Development of Transitional Rudiment and Growth Plate Cartilages and in the Maintenance of Permanent Cartilage Homeostasis.Int J Mol Sci. 2022 Feb 9;23(4):1934. doi: 10.3390/ijms23041934. Int J Mol Sci. 2022. PMID: 35216048 Free PMC article. Review.
-
HBP-A Attenuates Knee Osteoarthritis Progression via MLK3/P38/HDAC4 Axis-Mediated Dual Protection of Articular Cartilage and Quadriceps.J Cell Mol Med. 2025 May;29(9):e70577. doi: 10.1111/jcmm.70577. J Cell Mol Med. 2025. PMID: 40318007 Free PMC article.
-
What Are the Potential Roles of Nuclear Perlecan and Other Heparan Sulphate Proteoglycans in the Normal and Malignant Phenotype.Int J Mol Sci. 2021 Apr 23;22(9):4415. doi: 10.3390/ijms22094415. Int J Mol Sci. 2021. PMID: 33922532 Free PMC article. Review.
-
Wnt3a knockdown promotes collagen type II expression in rat chondrocytes.Exp Ther Med. 2022 Jun 17;24(2):526. doi: 10.3892/etm.2022.11453. eCollection 2022 Aug. Exp Ther Med. 2022. PMID: 35837029 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

