Positive feedback between retinoic acid and 2-phospho-L-ascorbic acid trisodium salt during somatic cell reprogramming
- PMID: 33000315
- PMCID: PMC7527398
- DOI: 10.1186/s13619-020-00057-1
Positive feedback between retinoic acid and 2-phospho-L-ascorbic acid trisodium salt during somatic cell reprogramming
Abstract
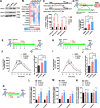
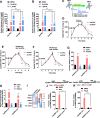
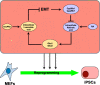
Retinoic acid (RA) and 2-phospho-L-ascorbic acid trisodium salt (AscPNa) promote the reprogramming of mouse embryonic fibroblasts to induced pluripotent stem cells. In the current studies, the lower abilities of RA and AscPNa to promote reprogramming in the presence of each other suggested that they may share downstream pathways at least partially. The hypothesis was further supported by the RNA-seq analysis which demonstrated a high-level overlap between RA-activated and AscPNa activated genes during reprogramming. In addition, RA upregulated Glut1/3, facilitated the membrane transportation of dehydroascorbic acid, the oxidized form of L-ascorbic acid, and subsequently maintained intracellular L-ascorbic acid at higher level and for longer time. On the other hand, AscPNa facilitated the mesenchymal-epithelial transition during reprogramming, downregulated key mesenchymal transcriptional factors like Zeb1 and Twist1, subsequently suppressed the expression of Cyp26a1/b1 which mediates the metabolism of RA, and sustained the intracellular level of RA. Furthermore, the different abilities of RA and AscPNa to induce mesenchymal-epithelial transition, pluripotency, and neuronal differentiation explain their complex contribution to reprogramming when used individually or in combination. Therefore, the current studies identified a positive feedback between RA and AscPNa, or possibility between vitamin A and C, and further explored their contributions to reprogramming.
Keywords: L-ascorbic acid; Mesenchymal-epithelial transition; Positive feedback; Reprogramming; Retinoic acid.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Targeted expression profiling reveals distinct stages of early canine fibroblast reprogramming are regulated by 2-oxoglutarate hydroxylases.Stem Cell Res Ther. 2020 Dec 9;11(1):528. doi: 10.1186/s13287-020-02047-1. Stem Cell Res Ther. 2020. PMID: 33298190 Free PMC article.
-
Incomplete cellular reprogramming of colorectal cancer cells elicits an epithelial/mesenchymal hybrid phenotype.J Biomed Sci. 2018 Jul 19;25(1):57. doi: 10.1186/s12929-018-0461-1. J Biomed Sci. 2018. PMID: 30025541 Free PMC article.
-
Unraveling the 2,3-diketo-L-gulonic acid-dependent and -independent impacts of L-ascorbic acid on somatic cell reprogramming.Cell Biosci. 2023 Nov 30;13(1):218. doi: 10.1186/s13578-023-01160-x. Cell Biosci. 2023. PMID: 38037169 Free PMC article.
-
Current understanding and future perspectives of the roles of sirtuins in the reprogramming and differentiation of pluripotent stem cells.Exp Biol Med (Maywood). 2018 Mar;243(6):563-575. doi: 10.1177/1535370218759636. Exp Biol Med (Maywood). 2018. PMID: 29557214 Free PMC article. Review.
-
Retinoic acid signaling and mouse embryonic stem cell differentiation: Cross talk between genomic and non-genomic effects of RA.Biochim Biophys Acta. 2015 Jan;1851(1):66-75. doi: 10.1016/j.bbalip.2014.04.003. Epub 2014 Apr 24. Biochim Biophys Acta. 2015. PMID: 24768681 Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
