Development and content validation of the Pediatric Oral Medicines Acceptability Questionnaires (P-OMAQ): patient-reported and caregiver-reported outcome measures
- PMID: 33000327
- PMCID: PMC7527387
- DOI: 10.1186/s41687-020-00246-1
Development and content validation of the Pediatric Oral Medicines Acceptability Questionnaires (P-OMAQ): patient-reported and caregiver-reported outcome measures
Abstract
Background: Evolving regulatory guidelines recommend routine assessment of the acceptability of pediatric oral medicines throughout clinical development processes. However, such assessment is problematic owing to a lack of standard methods or criteria that define acceptability for children and their caregivers. This research aimed to identify the attributes of acceptability for targeted oral formulation types that are important to children, and to develop content-valid patient- and caregiver-reported outcome acceptability measures for use in the context of clinical drug development.
Methods: A concept-focused literature review and two advisory panel meetings involving researchers, clinicians, and measurement scientists were conducted to identify acceptability attributes that may be relevant to children taking targeted oral medicine formulations. The Pediatric Oral Medicines Acceptability Questionnaires (P-OMAQs), including patient (P-OMAQ-P) and caregiver (P-OMAQ-C) versions, were drafted to assess these attributes. Qualitative concept elicitation (CE) and cognitive debriefing (CD) patient and caregiver interviews were conducted to confirm key acceptability attribute concepts for measurement and to evaluate patient and caregiver ability to understand and respond to the questions.
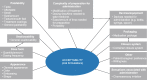
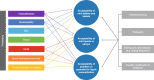
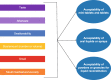
Results: A full-text review of 40 articles identified 24 acceptability attributes that were categorized into 10 overarching domains and organized into a preliminary conceptual model. Feedback from the advisory panel refined the preliminary model. In total, 14 attributes were reported during the CE phase of the interviews (n = 23 pediatric patients, n = 13 caregivers); six attributes were included in the final model. The draft P-OMAQ was refined over four waves of CD interviews (n = 31 pediatric patients, n = 48 caregivers). The final version of the P-OMAQ-P is a 12-item questionnaire designed for young people aged 8-17 years. The P-OMAQ-C is a 19-item questionnaire designed for adult caregivers of young people aged 6 months to 17 years. There are two versions of each questionnaire: one with a 24-h recall period and one with a 7-day recall period. All items are answered on a 5-point numerical rating scale.
Conclusions: This research supports the content validity of the patient and caregiver versions of the P-OMAQ. Both questionnaires appropriately assess the acceptability of oral medicine formulations from the perspective of pediatric patients and their caregivers.
Keywords: Acceptability; Caregivers; Clinical outcome assessments; Cognitive debriefing; Concept elicitation; Oral formulations; Pediatric oral medicines; Questionnaires; Systematic literature review.
Conflict of interest statement
DMT-B, AY and MKr are employed by Adelphi Values, which has received funding from Sanofi to conduct the qualitative research reported in this paper. EC, EM and MKe were paid employees of Adelphi Values at the time of this study. KA, NV, and GC are paid employees of Sanofi. MR was a paid employee of Sanofi at the time of this study. KA and MR are also stockholders of Sanofi. Editorial support, funded by Sanofi US Services Inc., was provided by Alice Fodder, Alistair Ray, and Jamie Singer of PharmaGenesis Oxford Central, Oxford, UK. Adelphi Values conducted the qualitative research, funded by Sanofi US Services Inc.
Figures
Similar articles
-
Development and Psychometric Evaluation of the Life Interference Questionnaire for Growth Hormone Deficiency (LIQ-GHD) to Assess Growth Hormone Injection Burden in Children and Adults.Patient. 2020 Jun;13(3):289-306. doi: 10.1007/s40271-019-00405-7. Patient. 2020. PMID: 31956960 Free PMC article.
-
Assessing asthma symptoms in children: qualitative research supporting the development of the Pediatric Asthma Diary-Child (PAD-C) and Pediatric Asthma Diary-Observer (PAD-O).J Patient Rep Outcomes. 2023 Oct 20;7(1):104. doi: 10.1186/s41687-023-00639-y. J Patient Rep Outcomes. 2023. PMID: 37863864 Free PMC article.
-
Development of the Patient- and Observer-Reported PRUCISION Instruments to Assess Pruritus and Sleep Disturbance in Pediatric Patients with Cholestatic Liver Diseases.Adv Ther. 2022 Nov;39(11):5126-5143. doi: 10.1007/s12325-022-02261-8. Epub 2022 Sep 6. Adv Ther. 2022. PMID: 36066744 Free PMC article. Review.
-
Key measurement concepts and appropriate clinical outcome assessments in pediatric achondroplasia clinical trials.Orphanet J Rare Dis. 2022 May 7;17(1):182. doi: 10.1186/s13023-022-02333-6. Orphanet J Rare Dis. 2022. PMID: 35525989 Free PMC article.
-
Patient Acceptability and Preferences for Solid Oral Dosage Form Drug Product Attributes: A Scoping Review.Patient Prefer Adherence. 2024 Jun 21;18:1281-1297. doi: 10.2147/PPA.S443213. eCollection 2024. Patient Prefer Adherence. 2024. PMID: 38919378 Free PMC article.
Cited by
-
ReNeu: A Pivotal, Phase IIb Trial of Mirdametinib in Adults and Children With Symptomatic Neurofibromatosis Type 1-Associated Plexiform Neurofibroma.J Clin Oncol. 2025 Feb 20;43(6):716-729. doi: 10.1200/JCO.24.01034. Epub 2024 Nov 8. J Clin Oncol. 2025. PMID: 39514826 Free PMC article. Clinical Trial.
-
Questionnaire Study to Investigate the Preferences of Children, Parents, and Healthcare Professionals for Different Formulations of Oral Medicinal Products.Pharmaceutics. 2024 Apr 8;16(4):515. doi: 10.3390/pharmaceutics16040515. Pharmaceutics. 2024. PMID: 38675176 Free PMC article.
-
Understanding data visualization techniques in qualitative studies used to develop and validate patient-reported outcome measures: a targeted literature review.Qual Life Res. 2025 Jul;34(7):2031-2048. doi: 10.1007/s11136-025-03964-5. Epub 2025 Apr 25. Qual Life Res. 2025. PMID: 40279025 Review.
-
Acceptability of Prednisolone in an Open-Label Randomised Cross-Over Study-Focus on Formulation in Children.Children (Basel). 2022 Aug 16;9(8):1236. doi: 10.3390/children9081236. Children (Basel). 2022. PMID: 36010126 Free PMC article.
-
Caregiver acceptability of seasonal malaria chemoprevention in two districts in the Upper West region, Ghana: a cross-sectional study.Malar J. 2025 Jan 14;24(1):14. doi: 10.1186/s12936-024-05169-6. Malar J. 2025. PMID: 39810150 Free PMC article.
References
-
- European Medicines Agency. (2013) Committee for medicinal products for human use, paediatric committee, guideline on pharmaceutical development of medicines for paediatric use. Available from: Http://www.Ema.Europa.Eu/docs/en_gb/document_library/scientific_guideline/2013/.... Accessed 29 June 2018.
-
- Liu F, Ranmal S, Batchelor HK, Orlu-Gul M, Ernest TB, Thomas IW, Flanagan T, Tuleu C. Patient-centred pharmaceutical design to improve acceptability of medicines: Similarities and differences in paediatric and geriatric populations. Drugs. 2014;74:1871–1889. doi: 10.1007/s40265-014-0297-2. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

