Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial
- PMID: 33000503
- PMCID: PMC7986183
- DOI: 10.1111/bjd.19573
Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial
Abstract
Background: Tralokinumab is a fully human monoclonal antibody that specifically neutralizes interleukin-13, a key driver of atopic dermatitis (AD).
Objectives: To evaluate the efficacy and safety of tralokinumab in combination with topical corticosteroids (TCS) in patients with moderate-to-severe AD who were candidates for systemic therapy.
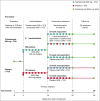
Methods: This was a double-blind, placebo plus TCS controlled phase III trial. Patients were randomized 2 : 1 to subcutaneous tralokinumab 300 mg or placebo every 2 weeks (Q2W) with TCS as needed over 16 weeks. Patients who achieved an Investigator's Global Assessment (IGA) score of 0/1 and/or 75% improvement in Eczema Area and Severity Index (EASI 75) at week 16 with tralokinumab were rerandomized 1 : 1 to tralokinumab Q2W or every 4 weeks (Q4W), with TCS as needed, for another 16 weeks.
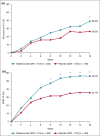
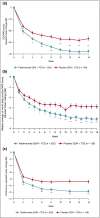
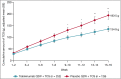
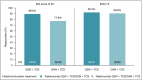
Results: At week 16, more patients treated with tralokinumab than with placebo achieved IGA 0/1: 38·9% vs. 26·2% [difference (95% confidence interval): 12·4% (2·9-21·9); P = 0·015] and EASI 75: 56·0% vs. 35·7% [20·2% (9·8-30·6); P < 0·001]. Of the patients who were tralokinumab responders at week 16, 89·6% and 92·5% of those treated with tralokinumab Q2W and 77·6% and 90·8% treated with tralokinumab Q4W maintained an IGA 0/1 and EASI 75 response at week 32, respectively. Among patients who did not achieve IGA 0/1 and EASI 75 with tralokinumab Q2W at 16 weeks, 30·5% and 55·8% achieved these endpoints, respectively, at week 32. The overall incidence of adverse events was similar across treatment groups.
Conclusions: Tralokinumab 300 mg in combination with TCS as needed was effective and well tolerated in patients with moderate-to-severe AD.
© 2020 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures
Comment in
-
Tralokinumab for atopic dermatitis: a promising new therapy.Br J Dermatol. 2021 Mar;184(3):386-387. doi: 10.1111/bjd.19699. Epub 2020 Dec 21. Br J Dermatol. 2021. PMID: 33347600 No abstract available.
References
-
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 2015; 66 (Suppl. 1):8–16. - PubMed
-
- Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. - PubMed
-
- Boguniewicz M, Alexis AF, Beck LA et al. Expert perspectives on management of moderate‐to‐severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract 2017; 5:1519–31. - PubMed
-
- Simpson EL, Bruin‐Weller M, Flohr C et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol 2017; 77:623–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

