Human-Recombinant-Elastin-Based Bioinks for 3D Bioprinting of Vascularized Soft Tissues
- PMID: 33000880
- PMCID: PMC7658039
- DOI: 10.1002/adma.202003915
Human-Recombinant-Elastin-Based Bioinks for 3D Bioprinting of Vascularized Soft Tissues
Abstract
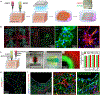
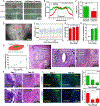
Bioprinting has emerged as an advanced method for fabricating complex 3D tissues. Despite the tremendous potential of 3D bioprinting, there are several drawbacks of current bioinks and printing methodologies that limit the ability to print elastic and highly vascularized tissues. In particular, fabrication of complex biomimetic structure that are entirely based on 3D bioprinting is still challenging primarily due to the lack of suitable bioinks with high printability, biocompatibility, biomimicry, and proper mechanical properties. To address these shortcomings, in this work the use of recombinant human tropoelastin as a highly biocompatible and elastic bioink for 3D printing of complex soft tissues is demonstrated. As proof of the concept, vascularized cardiac constructs are bioprinted and their functions are assessed in vitro and in vivo. The printed constructs demonstrate endothelium barrier function and spontaneous beating of cardiac muscle cells, which are important functions of cardiac tissue in vivo. Furthermore, the printed construct elicits minimal inflammatory responses, and is shown to be efficiently biodegraded in vivo when implanted subcutaneously in rats. Taken together, these results demonstrate the potential of the elastic bioink for printing 3D functional cardiac tissues, which can eventually be used for cardiac tissue replacement.
Keywords: GelMA; MeTro; bioprinting; cardiac tissue; elastic bioinks; elasticity; vascularized tissue.
© 2020 Wiley-VCH GmbH.
Figures


Similar articles
-
Peptide-dendrimer-reinforced bioinks for 3D bioprinting of heterogeneous and biomimetic in vitro models.Acta Biomater. 2023 Oct 1;169:243-255. doi: 10.1016/j.actbio.2023.08.008. Epub 2023 Aug 11. Acta Biomater. 2023. PMID: 37572980
-
Alginate-Based Bioinks for 3D Bioprinting and Fabrication of Anatomically Accurate Bone Grafts.Tissue Eng Part A. 2021 Sep;27(17-18):1168-1181. doi: 10.1089/ten.TEA.2020.0305. Epub 2021 Feb 26. Tissue Eng Part A. 2021. PMID: 33218292 Free PMC article.
-
Bioprinting 101: Design, Fabrication, and Evaluation of Cell-Laden 3D Bioprinted Scaffolds.Tissue Eng Part A. 2020 Mar;26(5-6):318-338. doi: 10.1089/ten.TEA.2019.0298. Tissue Eng Part A. 2020. PMID: 32079490 Free PMC article.
-
Advancing bioinks for 3D bioprinting using reactive fillers: A review.Acta Biomater. 2020 Sep 1;113:1-22. doi: 10.1016/j.actbio.2020.06.040. Epub 2020 Jul 2. Acta Biomater. 2020. PMID: 32622053 Review.
-
Recent Trends in Decellularized Extracellular Matrix Bioinks for 3D Printing: An Updated Review.Int J Mol Sci. 2019 Sep 18;20(18):4628. doi: 10.3390/ijms20184628. Int J Mol Sci. 2019. PMID: 31540457 Free PMC article. Review.
Cited by
-
Aqueous Two-Phase Enabled Low Viscosity 3D (LoV3D) Bioprinting of Living Matter.Adv Sci (Weinh). 2023 Mar;10(8):e2204609. doi: 10.1002/advs.202204609. Epub 2022 Dec 30. Adv Sci (Weinh). 2023. PMID: 36585374 Free PMC article.
-
Tailoring bioinks of extrusion-based bioprinting for cutaneous wound healing.Bioact Mater. 2022 Jan 29;17:178-194. doi: 10.1016/j.bioactmat.2022.01.024. eCollection 2022 Nov. Bioact Mater. 2022. PMID: 35386443 Free PMC article. Review.
-
1Biomaterial inks for extrusion-based 3D bioprinting: Property, classification, modification, and selection.Int J Bioprint. 2022 Dec 9;9(2):649. doi: 10.18063/ijb.v9i2.649. eCollection 2023. Int J Bioprint. 2022. PMID: 37065674 Free PMC article.
-
Light-based 3D bioprinting techniques for illuminating the advances of vascular tissue engineering.Mater Today Bio. 2024 Oct 2;29:101286. doi: 10.1016/j.mtbio.2024.101286. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39435375 Free PMC article. Review.
-
Thixotropic Hydrogels Composed of Self-Assembled Nanofibers of Double-Hydrophobic Elastin-Like Block Polypeptides.Int J Mol Sci. 2021 Apr 15;22(8):4104. doi: 10.3390/ijms22084104. Int J Mol Sci. 2021. PMID: 33921095 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

