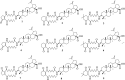Betulinic Acid-Azaprostanoid Hybrids: Synthesis and Pharmacological Evaluation as Anti-inflammatory Agents
- PMID: 33001006
- PMCID: PMC7499346
- DOI: 10.2174/1871523018666190426152049
Betulinic Acid-Azaprostanoid Hybrids: Synthesis and Pharmacological Evaluation as Anti-inflammatory Agents
Abstract
Background: Prevention and treatment of chronic inflammatory diseases require effective and low-toxic medicines. Molecular hybridization is an effective strategy to enhance the biological activity of new compounds. Triterpenoid scaffolds are in the focus of attention owing to their anti-inflammatory, antiviral, antiproliferative, and immunomodulatory activities. Heteroprostanoids have different pleiotropic effects in acute and chronic inflammatory processes.
Objective: The study aimed to develop structurally new and low toxic anti-inflammatory agents via hybridization of betulinic acid with azaprostanoic acids.
Methods: A series of betulinic acid-azaprostanoid hybrids was synthesized. The synthetic pathway included the transformation of betulin via Jones' oxidation into betulonic acid, reductive amination of the latter and coupling obtained by 3β-amino-3-deoxybetulinic acid with the 7- or 13-azaprostanoic acids and their homo analogues. The hybrids 1-9 were investigated in vivo on histamine-, formalin- and concanavalin A-induced mouse paw edema models and two models of pain - the acetic acid-induced abdominal writhing and the hotplate test. The hybrids were in vitro evaluated for cytotoxic activity on cancer (MCF7, U- 87 MG) and non-cancer humane cell lines.
Results: In the immunogenic inflammation model, the substances showed a pronounced anti-inflammatory effect, which was comparable to that of indomethacin. In the models of the exudative inflammation, none of the compounds displayed a statistically significant effect. The hybrids produced weak or moderate analgesic effects. All the agents revealed low cytotoxicity on human immortalized fibroblasts and cancer cell lines compared with 3β- amino-3-deoxybetulinic acid and doxorubicin.
Conclusion: The results indicate that the principal anti-inflammatory effect of hybrids is substantially provided with the triterpenoid scaffold and in some cases with the azaprostanoid scaffold, but the latter makes a significant contribution to reducing the toxicity of hybrids. Hybrid 1 is of interest as a potent low toxic agent against immune-mediated inflammation.
Keywords: Analgesic activity; anti-inflammatory activity; azaprostanoids; betulinic acid; cytotoxicity; hybrids..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
References
-
- Newman D.J., Cragg C.M. Natural product scaffolds of value in medicinal chemistry. In: Bräse S., editor. Privileged scaffolds in medicinal chemistry. Design, synthesis, evaluation. Cambridge, UK: Royal Society of Chemistry; 2016. pp. 348–378.
-
- Tolstikova T.G., Sorokina I.V., Tolstikov G.A., Tolstikov A.G., Flekhter O.B. [Biological activity and pharmacological prospects of lupane terpenoids: I. Natural lupane derivatives]. Bioorg. Khim. 2006;32(1):42–55. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources