Feasibility and Acceptability of Wearable Sleep Electroencephalogram Device Use in Adolescents: Observational Study
- PMID: 33001035
- PMCID: PMC7563632
- DOI: 10.2196/20590
Feasibility and Acceptability of Wearable Sleep Electroencephalogram Device Use in Adolescents: Observational Study
Abstract
Background: Adolescence is an important life stage for the development of healthy behaviors, which have a long-lasting impact on health across the lifespan. Sleep undergoes significant changes during adolescence and is linked to physical and psychiatric health; however, sleep is rarely assessed in routine health care settings. Wearable sleep electroencephalogram (EEG) devices may represent user-friendly methods for assessing sleep among adolescents, but no studies to date have examined the feasibility and acceptability of sleep EEG wearables in this age group.
Objective: The goal of the research was to investigate the feasibility and acceptability of sleep EEG wearable devices among adolescents aged 11 to 17 years.
Methods: A total of 104 adolescents aged 11 to 17 years participated in 7 days of at-home sleep recording using a self-administered wearable sleep EEG device (Zmachine Insight+, General Sleep Corporation) as well as a wristworn actigraph. Feasibility was assessed as the number of full nights of successful recording completed by adolescents, and acceptability was measured by the wearable acceptability survey for sleep. Feasibility and acceptability were assessed separately for the sleep EEG device and wristworn actigraph.
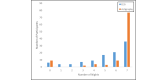
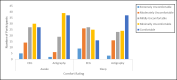
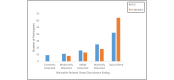
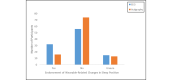
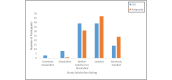
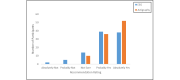
Results: A total of 94.2% (98/104) of adolescents successfully recorded at least 1 night of data using the sleep EEG device (mean number of nights 5.42; SD 1.71; median 6, mode 7). A total of 81.6% (84/103) rated the comfort of the device as falling in the comfortable to mildly uncomfortable range while awake. A total of 40.8% (42/103) reported typical sleep while using the device, while 39.8% (41/103) indicated minimal to mild device-related sleep disturbances. A minority (32/104, 30.8%) indicated changes in their sleep position due to device use, and very few (11/103, 10.7%) expressed dissatisfaction with their experience with the device. A similar pattern was observed for the wristworn actigraph device.
Conclusions: Wearable sleep EEG appears to represent a feasible, acceptable method for sleep assessment among adolescents and may have utility for assessing and treating sleep disturbances at a population level. Future studies with adolescents should evaluate strategies for further improving usability of such devices, assess relationships between sleep EEG-derived metrics and health outcomes, and investigate methods for incorporating data from these devices into emerging digital interventions and applications.
Trial registration: ClinicalTrials.gov NCT03843762; https://clinicaltrials.gov/ct2/show/NCT03843762.
Keywords: EEG; acceptability; actigraphy; adolescents; feasibility; mHealth; sleep; tolerability; wearable.
©Jessica R Lunsford-Avery, Casey Keller, Scott H Kollins, Andrew D Krystal, Leah Jackson, Matthew M Engelhard. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 01.10.2020.
Conflict of interest statement
Conflicts of Interest: Authors do not have any conflicts of interest to report in regard to the devices used in this study. ADK has received research funding from Janssen Pharmaceuticals, Axsome Pharmaceutics, Reveal Biosensors, The Ray and Dagmar Dolby Family Fund, and the National Institutes of Health. He has received consulting fees from Adare, Axsome Therapeutics, Big Data, Eisai, Evecxia, Ferring Pharmaceuticals, Galderma, Harmony Biosciences, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Millennium Pharmaceuticals, Merck, Neurocrine Biosciences, Pernix, Otsuka Pharmaceuticals, Sage, and Takeda. Authors JRLA, CEK, SHK, LJ, and MME do not have any conflicts of interest to report.
Figures
References
-
- Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018 Aug;67:55–65. doi: 10.1016/j.adolescence.2018.06.001. http://europepmc.org/abstract/MED/29908393 - DOI - PMC - PubMed
-
- Garbarino S, Lanteri P, Durando P, Magnavita N, Sannita WG. Co-morbidity, mortality, quality of life and the healthcare/welfare/social costs of disordered sleep: a rapid review. Int J Environ Res Public Health. 2016 Aug 18;13(8) doi: 10.3390/ijerph13080831. http://www.mdpi.com/resolver?pii=ijerph13080831 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

