Association of Pretreatment With P2Y12 Receptor Antagonists Preceding Percutaneous Coronary Intervention in Non-ST-Segment Elevation Acute Coronary Syndromes With Outcomes
- PMID: 33001202
- PMCID: PMC7530628
- DOI: 10.1001/jamanetworkopen.2020.18735
Association of Pretreatment With P2Y12 Receptor Antagonists Preceding Percutaneous Coronary Intervention in Non-ST-Segment Elevation Acute Coronary Syndromes With Outcomes
Abstract
Importance: Pretreatment of patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS) with P2Y12 receptor antagonists is a common practice despite the lack of definite evidence for its benefit.
Objective: To investigate the association of P2Y12 receptor antagonist pretreatment vs no pretreatment with mortality, stent thrombosis, and in-hospital bleeding in patients with NSTE-ACS undergoing percutaneous coronary intervention (PCI).
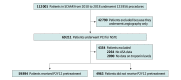
Design, setting, and participants: This cohort study used prospective data from the Swedish Coronary Angiography and Angioplasty Registry of 64 857 patients who underwent procedures between 2010 and 2018. All patients who underwent PCI owing to NSTE-ACS in Sweden were stratified by whether they were pretreated with P2Y12 receptor antagonists. Associations of pretreatment with P2Y12 receptor antagonists with the risks of adverse outcomes were investigated using instrumental variable analysis and propensity score matching. Data were analyzed from March to June 2019.
Exposures: Pretreatment with P2Y12 receptor antagonists.
Main outcomes and measures: The primary end point was all-cause mortality within 30 days. Secondary end points were 1-year mortality, stent thrombosis within 30 days, and in-hospital bleeding.
Results: In total, 64 857 patients (mean [SD] age, 64.7 [10.9] years; 46 809 [72.2%] men) were included. A total of 59 894 patients (92.4%) were pretreated with a P2Y12 receptor antagonist, including 27 867 (43.7%) pretreated with clopidogrel, 34 785 (54.5%) pretreated with ticagrelor, and 1148 (1.8%) pretreated with prasugrel. At 30 days, there were 971 deaths (1.5%) and 101 definite stent thromboses (0.2%) in the full cohort. Pretreatment was not associated with better survival at 30 days (odds ratio [OR], 1.17; 95% CI, 0.66-2.11; P = .58), survival at 1 year (OR, 1.34; 95% CI, 0.77-2.34; P = .30), or decreased stent thrombosis (OR, 0.81; 95% CI, 0.42-1.55; P = .52). However, pretreatment was associated with increased risk of in-hospital bleeding (OR, 1.49; 95% CI, 1.06-2.12; P = .02).
Conclusions and relevance: This cohort study found that pretreatment of patients with NSTE-ACS with P2Y12 receptor antagonists was not associated with improved clinical outcomes but was associated with increased risk of bleeding. These findings support the argument that pretreatment with P2Y12 receptor antagonists should not be routinely used in patients with NSTE-ACS.
Conflict of interest statement
Figures
References
-
- Hamm CW, Bassand JP, Agewall S, et al. ; ESC Committee for Practice Guidelines . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(23):2999-3054. doi:10.1093/eurheartj/ehr236 - DOI - PubMed
-
- Amsterdam EA, Wenger NK, Brindis RG, et al. . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228. doi:10.1016/j.jacc.2014.09.017 - DOI - PubMed
-
- Steinhubl SR, Berger PB, Mann JT III, et al. ; CREDO Investigators; Clopidogrel for the Reduction of Events During Observation . Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411-2420. doi:10.1001/jama.288.19.2411 - DOI - PubMed
-
- Mehta SR, Yusuf S, Peters RJ, et al. ; Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators . Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527-533. doi:10.1016/S0140-6736(01)05701-4 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous