Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset
- PMID: 33001206
- PMCID: PMC7714093
- DOI: 10.1182/blood.2020008367
Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset
Abstract
There is a
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
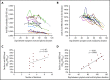
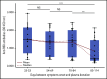
Comment in
-
Catch those antibodies before they fall.Blood. 2020 Nov 26;136(22):2489-2490. doi: 10.1182/blood.2020008768. Blood. 2020. PMID: 33242330 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

