Driving performance and neurocognitive skills of long-term users of sedating antidepressants
- PMID: 33001492
- PMCID: PMC7816239
- DOI: 10.1002/hup.2762
Driving performance and neurocognitive skills of long-term users of sedating antidepressants
Abstract
Objective: To assess driving performance and neurocognitive skills of long-term users of sedating antidepressants, in comparison to healthy controls.
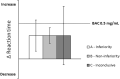
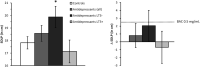
Methods: Thirty-eight long-term (>6 months) users of amitriptyline (n = 13) and mirtazapine (n = 25) were compared to 65 healthy controls. Driving performance was assessed using a 1-h standardised highway driving test in actual traffic, with road-tracking error (standard deviation of lateral position [SDLP]) being the primary measure. Secondary measures included neurocognitive tasks related to driving. Performance differences between groups were compared to those of blood alcohol concentrations of 0.5 mg/ml to determine clinical relevance.
Results: Compared to controls, mean increase in SDLP of all antidepressant users was not significant, nor clinically relevant (+0.75 cm, 95% CI: -0.83 cm; +2.33 cm). However, users treated less than 3 years (n = 20) did show a significant and clinically relevant increase in SDLP (+2.05 cm). No significant effects were observed on neurocognitive tasks for any user group, although large individual differences were present. Most results from neurocognitive tests were inconclusive, while a few parameters confirmed non-inferiority for users treated longer than 3 years.
Conclusion: The impairing effects of antidepressant treatment on driving performance and neurocognition mitigate over time following long-term use of 3 years.
Keywords: antidepressants; driving performance; long-term use; neurocognition; on-the-road driving.
© 2020 The Authors. Human Psychopharmacology: Clinical and Experimental published by John Wiley & Sons Ltd.
Conflict of interest statement
J.C. Verster has received grants from Janssen, Nutricia, Red Bull, Sequential, Takeda, and acted as a consultant/advisor for 82Labs, Canadian Beverage Association, Centraal Bureau Drogisterij bedrijven, Clinilabs, Coleman Frost, Danone, Deenox, Eisai, Janssen, Jazz, Purdue, Red Bull, Sanofi‐Aventis, Sen‐Jam Pharmaceutical, Sepracor, Takeda, Transcept, Trimbos Institute, Vital Bevrages and ZBiotics. A. Vermeeren and J.G. Ramaekers have received funding over the last 4 years from pharmaceutical companies (Eisai, Jazz, Merck and Transcept).
Figures

References
-
- Arroll, B. , Macgillivray, S. , Ogston, S. , Reid, I. , Sullivan, F. , Williams, B. , & Crombie, I. (2005). Efficacy and tolerability of tricyclic antidepressants and SSRIs compared with placebo for treatment of depression in primary care: A meta‐analysis. The Annals of Family Medicine, 3(5), 449–456. - PMC - PubMed
-
- Barbone, F. , McMahon, A. , & Davey, P. (1998). Use of benzodiazepines increased road traffic accidents whereas use of tricyclic antidepressants and SSRIs did not. Lancet, 24, 1331–1336. - PubMed
-
- Bauer, M. , Pfennig, A. , Severus, E. , Whybrow, P. C. , Angst, J. , & Moeller, H. J. (2013). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: Update 2013 on the acute and continuation treatment of unipolar depressive disorders. World Journal of Biological Psychiatry, 14(5), 334–385. - PubMed
-
- Beck, A. T. , Steer, R. A. , & Carbin, M. G. (1988). Psychometric properties of the Beck Depression Inventory: Twenty‐five years of evaluation. Clinical Psychology Review, 8(1), 77–100.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

