Supervised segmentation framework for evaluation of diffusion tensor imaging indices in skeletal muscle
- PMID: 33001508
- PMCID: PMC7757256
- DOI: 10.1002/nbm.4406
Supervised segmentation framework for evaluation of diffusion tensor imaging indices in skeletal muscle
Abstract
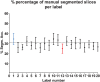
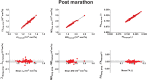
Diffusion tensor imaging (DTI) is becoming a relevant diagnostic tool to understand muscle disease and map muscle recovery processes following physical activity or after injury. Segmenting all the individual leg muscles, necessary for quantification, is still a time-consuming manual process. The purpose of this study was to evaluate the impact of a supervised semi-automatic segmentation pipeline on the quantification of DTI indices in individual upper leg muscles. Longitudinally acquired MRI datasets (baseline, post-marathon and follow-up) of the upper legs of 11 subjects were used in this study. MR datasets consisted of a DTI and Dixon acquisition. Semi-automatic segmentations for the upper leg muscles were performed using a transversal propagation approach developed by Ogier et al on the out-of-phase Dixon images at baseline. These segmentations were longitudinally propagated for the post-marathon and follow-up time points. Manual segmentations were performed on the water image of the Dixon for each of the time points. Dice similarity coefficients (DSCs) were calculated to compare the manual and semi-automatic segmentations. Bland-Altman and regression analyses were performed, to evaluate the impact of the two segmentation methods on mean diffusivity (MD), fractional anisotropy (FA) and the third eigenvalue (λ3 ). The average DSC for all analyzed muscles over all time points was 0.92 ± 0.01, ranging between 0.48 and 0.99. Bland-Altman analysis showed that the 95% limits of agreement for MD, FA and λ3 ranged between 0.5% and 3.0% for the transversal propagation and between 0.7% and 3.0% for the longitudinal propagations. Similarly, regression analysis showed good correlation for MD, FA and λ3 (r = 0.99, p < 60; 0.0001). In conclusion, the supervised semi-automatic segmentation framework successfully quantified DTI indices in the upper-leg muscles compared with manual segmentation while only requiring manual input of 30% of the slices, resulting in a threefold reduction in segmentation time.
Keywords: applications; diffusion tensor imaging (DTI); methods and engineering; muscle; musculoskeletal; post-acquisition processing; quantitation.
© 2020 The Authors. NMR in Biomedicine published by John Wiley & Sons Ltd.
Figures


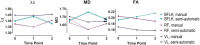



References
-
- Saifuddin A, Tyler P, Hargunani R. Musculoskeletal MRI. CRC Press; 2016.
-
- Froeling M, Nederveen AJ, Heijtel DF, et al. Diffusion‐tensor MRI reveals the complex muscle architecture of the human forearm. J Magn Reson Imaging. 2012;36(1):237‐248. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

