Predicting Nitrogen-Based Families of Compounds: Transition-Metal Guanidinates TCN3 (T=V, Nb, Ta) and Ortho-Nitrido Carbonates T'2 CN4 (T'=Ti, Zr, Hf)
- PMID: 33001558
- PMCID: PMC7821139
- DOI: 10.1002/anie.202011196
Predicting Nitrogen-Based Families of Compounds: Transition-Metal Guanidinates TCN3 (T=V, Nb, Ta) and Ortho-Nitrido Carbonates T'2 CN4 (T'=Ti, Zr, Hf)
Abstract
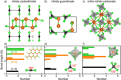
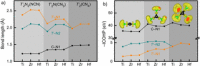
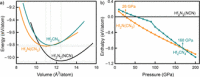
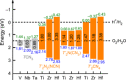
Due to its unsurpassed capability to engage in various sp hybridizations or orbital mixings, carbon may contribute in expanding solid-state nitrogen chemistry by allowing for different complex anions, such as the known NCN2- carbodiimide unit, the so far unknown CN3 5- guanidinate anion, and the likewise unknown CN4 8- ortho-nitrido carbonate (onc) entity. Because the latter two complex anions have never been observed before, we have chemically designed them using first-principles structural searches, and we here predict the first hydrogen-free guanidinates TCN3 (T=V, Nb, Ta) and ortho-nitrido carbonates T'2 CN4 (T'=Ti, Zr, Hf) being mechanically stable at normal pressure; the latter should coexist as solid solutions with the stoichiometrically identical nitride carbodiimides and nitride guanidinates. We also suggest favorable exothermic reactions as useful signposts for eventual synthesis, and we trust that the decay of the novel compounds is unlikely due to presumably large kinetic activation barriers (C-N bond breaking) and quite substantial Madelung energies stabilizing the highly charged complex anions. While chemical-bonding analysis reveals the novel CN4 8- to be more covalent compared to NCN2- and CN3 5- within related compounds, further electronic-structure data of onc phases hint at their physicochemical potential in terms of photoelectrochemical water splitting and nonlinear optics.
Keywords: carbodiimide; guanidinate; non-linear optics; ortho-nitrido carbonate; water splitting.
© 2020 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- None
-
- Holleman A. F., Wiberg N., Wiberg E., Lehrbuch der Anorganischen Chemie, Walter de Gruyter, New York, 2007;
-
- Greenwood N. N., Earnshaw A., Chemistry of the Elements, Second Edition, Butterworth-Heinemann, Oxford, 1997;
-
- Dossett J. L., Totten G. E., ASM Handbook: Steel Heat Treating Fundamentals and Processes, ASM International, Maerials Park, 2013;
-
- Yamanaka S., Hotehama K.-I., Kawaji H., Nature 1998, 392, 580–582;
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

