Application of a plasmin generation assay to define pharmacodynamic effects of tranexamic acid in women undergoing cesarean delivery
- PMID: 33001565
- PMCID: PMC7875467
- DOI: 10.1111/jth.15114
Application of a plasmin generation assay to define pharmacodynamic effects of tranexamic acid in women undergoing cesarean delivery
Abstract
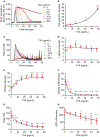
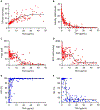
Essentials Tranexamic acid (TXA) is an antifibrinolytic drug used to reduce bleeding. Assaying plasmin generation (PG) in plasma detects clinically relevant TXA levels in vitro and ex vivo. 3.1-16.2 µg/mL TXA half-maximally inhibits PG in plasma from women undergoing cesarean delivery. PG velocity shows the strongest dose-relationship at low TXA concentrations (≤10 µg/mL). ABSTRACT: Background Tranexamic acid (TXA) is used to reduce bleeding. TXA inhibits plasmin(ogen) binding to fibrin and reduces fibrinolysis. TXA antifibrinolytic activity is typically measured by clot lysis assays; however, effects on plasmin generation (PG) are unclear due to a lack of tools to measure PG in plasma. Aims Develop an assay to measure PG kinetics in human plasma. Determine effects of TXA on PG and compare with fibrinolysis measured by rotational thromboelastometry (ROTEM). Methods We characterized effects of plasminogen, tissue plasminogen activator, fibrinogen, and α2 -antiplasmin on PG in vitro. We also studied effects of TXA on PG in plasma from 30 pregnant women administered intravenous TXA (5, 10, or 15 mg/kg) during cesarean delivery. PG was measured by calibrated fluorescence. PG parameters were compared with TXA measured by mass spectrometry and ROTEM of whole blood. Results The PG assay is specific for plasmin and sensitive to tissue plasminogen activator, fibrin(ogen), and α2 -antiplasmin. Addition of TXA to plasma in vitro dose dependently prolonged the clot lysis time and delayed and reduced PG. For all doses of TXA administered intravenously, the PG assay detected delayed time-to-peak (≤3 hours) and reduced the velocity, peak, and endogenous plasmin potential (≤24 hours) in plasma samples obtained after infusion. The PG time-to-peak, velocity, and peak correlated significantly with TXA concentration and showed less variability than the ROTEM lysis index at 30 minutes or maximum lysis. Conclusions The PG assay detects pharmacologically relevant concentrations of TXA administered in vitro and in vivo, and demonstrates TXA-mediated inhibition of PG in women undergoing cesarean delivery.
Keywords: fibrin; fibrinolysis; plasmin; pregnancy; tranexamic acid.
© 2020 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
CONFLICT OF INTEREST
Dr. Miszta and Dr. de Laat are employed by Synapse Research Institute, a not-for-profit member of the STAGO Diagnostic group that produces calibrated automated thrombography for thrombin generation measurements in plasma. Synapse Research Institute holds the patent on calibrated plasmin generation. The ROTEM was provided by the manufacturer, which did not provide any input on the study design or data interpretation. None of the other authors have relevant potential conflict of interest.
Figures


References
-
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–e333. - PubMed
-
- Sperzel M, Huetter J. Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. J Thromb Haemost. 2007;5(10):2113–2118. - PubMed
-
- Silva MM, Thelwell C, Williams SC, Longstaff C. Regulation of fibrinolysis by C-terminal lysines operates through plasminogen and plasmin but not tissue-type plasminogen activator. J Thromb Haemost. 2012;10(11):2354–2360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

