Dissecting the Cellular Mechanism of Prostacyclin Analog Iloprost in Reversing Vascular Dysfunction in Scleroderma
- PMID: 33001586
- PMCID: PMC7914149
- DOI: 10.1002/art.41536
Dissecting the Cellular Mechanism of Prostacyclin Analog Iloprost in Reversing Vascular Dysfunction in Scleroderma
Abstract
Objective: Intravenous iloprost improves Raynaud's phenomenon (RP) and promotes healing of digital ulcers in systemic sclerosis (SSc; scleroderma). Despite a short half-life, its clinical efficacy lasts weeks. Endothelial adherens junctions, which are formed by VE-cadherin clustering between endothelial cells (ECs), regulate endothelial properties including barrier function, endothelial-to-mesenchymal transition (EndoMT), and angiogenesis. We undertook this study to investigate the hypothesis that junctional disruption contributes to vascular dysfunction in SSc, and that the protective effect of iloprost is mediated by strengthening of those junctions.
Methods: Dermal ECs from SSc patients and healthy controls were isolated. The effect of iloprost on ECs was examined using immunofluorescence, permeability assays, Matrigel tube formation, and quantitative polymerase chain reaction.
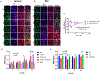
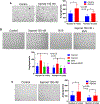
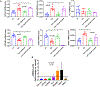
Results: Adherens junctions in SSc were disrupted compared to normal ECs, as indicated by reduced levels of VE-cadherin and increased permeability in SSc ECs (P < 0.05). Iloprost increased VE-cadherin clustering at junctions and restored junctional levels of VE-cadherin in SSc ECs (mean ± SD 37.3 ± 4.3 fluorescence units) compared to normal ECs (mean ± SD 29.7 ± 3.4 fluorescence units; P < 0.05), after 2 hours of iloprost incubation. In addition, iloprost reduced permeability of monolayers, increased tubulogenesis, and blocked EndoMT in both normal and SSc ECs (n ≥ 3; P < 0.05). The effects in normal ECs were inhibited by a function-blocking antibody that prevents junctional clustering of VE-cadherin.
Conclusion: Our data suggest that the long-lasting effects of iloprost reflect its ability to stabilize adherens junctions, resulting in increased tubulogenesis and barrier function and reduced EndoMT. These findings provide a mechanistic basis for the use of iloprost in treating SSc patients with RP and digital ulcers.
© 2020, American College of Rheumatology.
Conflict of interest statement
Figures

References
-
- Olschewski H, Rose F, Schermuly R, Ghofrani HA, Enke B, Olschewski A, et al. Prostacyclin and its analogues in the treatment of pulmonary hypertension. Pharmacology & therapeutics. 2004;102(2):139–53. - PubMed
-
- Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327–39. - PubMed
-
- Baumer Y, Drenckhahn D, Waschke J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem Cell Biol. 2008;129(6):765–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

