Protein tyrosine phosphatase nonreceptor type 2 controls colorectal cancer development
- PMID: 33001862
- PMCID: PMC7773375
- DOI: 10.1172/JCI140281
Protein tyrosine phosphatase nonreceptor type 2 controls colorectal cancer development
Abstract
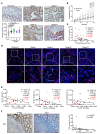
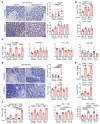
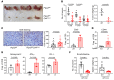
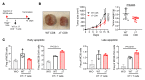
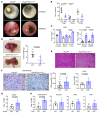
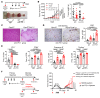
Protein tyrosine phosphatase nonreceptor type 2 (PTPN2) recently emerged as a promising cancer immunotherapy target. We set out to investigate the functional role of PTPN2 in the pathogenesis of human colorectal carcinoma (CRC), as its role in immune-silent solid tumors is poorly understood. We demonstrate that in human CRC, increased PTPN2 expression and activity correlated with disease progression and decreased immune responses in tumor tissues. In particular, stage II and III tumors displayed enhanced PTPN2 protein expression in tumor-infiltrating T cells, and increased PTPN2 levels negatively correlated with expression of PD-1, CTLA4, STAT1, and granzyme A. In vivo, T cell- and DC-specific PTPN2 deletion reduced tumor burden in several CRC models by promoting CD44+ effector/memory T cells, as well as CD8+ T cell infiltration and cytotoxicity in the tumor. In direct relevance to CRC treatment, T cell-specific PTPN2 deletion potentiated anti-PD-1 efficacy and induced antitumor memory formation upon tumor rechallenge in vivo. Our data suggest a role for PTPN2 in suppressing antitumor immunity and promoting tumor development in patients with CRC. Our in vivo results identify PTPN2 as a key player in controlling the immunogenicity of CRC, with the strong potential to be exploited for cancer immunotherapy.
Keywords: Cancer immunotherapy; Colorectal cancer; Gastroenterology; Oncology; T cells.
Conflict of interest statement
Figures


Similar articles
-
PTPN2 in the Immunity and Tumor Immunotherapy: A Concise Review.Int J Mol Sci. 2022 Sep 2;23(17):10025. doi: 10.3390/ijms231710025. Int J Mol Sci. 2022. PMID: 36077422 Free PMC article. Review.
-
Small-molecule PTPN2 Inhibitors Sensitize Resistant Melanoma to Anti-PD-1 Immunotherapy.Cancer Res Commun. 2023 Jan 24;3(1):119-129. doi: 10.1158/2767-9764.CRC-21-0186. eCollection 2023 Jan. Cancer Res Commun. 2023. PMID: 36968224 Free PMC article.
-
PTPN2 restrains CD8⁺ T cell responses after antigen cross-presentation for the maintenance of peripheral tolerance in mice.J Autoimmun. 2014 Sep;53:105-14. doi: 10.1016/j.jaut.2014.05.008. Epub 2014 Jul 2. J Autoimmun. 2014. PMID: 24997008
-
Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8+ regulatory T cells.Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2102950118. doi: 10.1073/pnas.2102950118. Proc Natl Acad Sci U S A. 2021. PMID: 34006646 Free PMC article.
-
Colorectal Cancer Immune Infiltrates: Significance in Patient Prognosis and Immunotherapeutic Efficacy.Front Immunol. 2020 May 28;11:1052. doi: 10.3389/fimmu.2020.01052. eCollection 2020. Front Immunol. 2020. PMID: 32547556 Free PMC article. Review.
Cited by
-
A small molecule inhibitor of PTP1B and PTPN2 enhances T cell anti-tumor immunity.Nat Commun. 2023 Jul 27;14(1):4524. doi: 10.1038/s41467-023-40170-8. Nat Commun. 2023. PMID: 37500611 Free PMC article.
-
Tumor microenvironment responsive nano-herb and CRISPR delivery system for synergistic chemotherapy and immunotherapy.J Nanobiotechnology. 2024 Jun 19;22(1):346. doi: 10.1186/s12951-024-02571-9. J Nanobiotechnology. 2024. PMID: 38898493 Free PMC article.
-
Protein tyrosine phosphatase nonreceptor 2: A New biomarker for digestive tract cancers.World J Gastrointest Oncol. 2025 Feb 15;17(2):100546. doi: 10.4251/wjgo.v17.i2.100546. World J Gastrointest Oncol. 2025. PMID: 39958541 Free PMC article.
-
PTPN2 in the Immunity and Tumor Immunotherapy: A Concise Review.Int J Mol Sci. 2022 Sep 2;23(17):10025. doi: 10.3390/ijms231710025. Int J Mol Sci. 2022. PMID: 36077422 Free PMC article. Review.
-
The Inhibitors of Protein Tyrosine Phosphatase Nonreceptor Type 2 (PTPN2) as Potential Enhancers of Cancer Immunotherapy and Type 1 (PTPN1) as Treatment of Metabolic Diseases.ACS Med Chem Lett. 2021 Dec 27;13(1):19-21. doi: 10.1021/acsmedchemlett.1c00678. eCollection 2022 Jan 13. ACS Med Chem Lett. 2021. PMID: 35059117 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

