Induction of oxidative stress biomarkers following whole-body irradiation in mice
- PMID: 33002096
- PMCID: PMC7529313
- DOI: 10.1371/journal.pone.0240108
Induction of oxidative stress biomarkers following whole-body irradiation in mice
Abstract
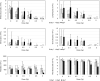
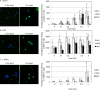
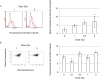
Dose assessment is an important issue for radiation emergency medicine to determine appropriate clinical treatment. Hematopoietic tissues are extremely vulnerable to radiation exposure. A decrease in blood cell count following radiation exposure is the first quantitative bio-indicator using hematological techniques. We further examined induction of oxidative stress biomarkers in residual lymphocytes to identify new biomarkers for dosimetry. In vivo whole-body radiation to mice exposed to 5 Gy significantly induces DNA double-strand breaks, which were visualized by γ-H2AX in mouse blood cells. Mouse blood smears and peripheral blood mononuclear cells (PBMC) isolated from irradiated mice were used for immunostaining for oxidative biomarkers, parkin or Nrf2. Parkin is the E3 ubiquitin ligase, which is normally localized in the cytoplasm, is relocated to abnormal mitochondria with low membrane potential (ΔΨm), where it promotes clearance via mitophagy. Nrf2 transcription factor controls the major cellular antioxidant responses. Both markers of oxidative stress were more sensitive and persistent over time than nuclear DNA damage. In conclusion, parkin and Nrf2 are potential biomarkers for use in radiation dosimetry. Identification of several biological markers which show different kinetics for radiation response is essential for radiation dosimetry that allows the assessment of radiation injury and efficacy of clinical treatment in emergency radiation incidents. Radiation-induced oxidative damage is useful not only for radiation dose assessment but also for evaluation of radiation risks on humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- International Atomic Energy Agency DoNS, Security I, Emergency Centre V (2011) Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies. International Atomic Energy Agency (IAEA). 247 p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

