Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies
- PMID: 33002134
- PMCID: PMC7556134
- DOI: 10.1182/bloodadvances.2020002118
Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies
Abstract
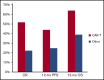
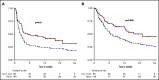
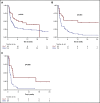
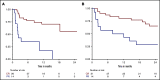
The prognosis of patients with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) is poor. Chimeric antigen receptor (CAR) T-cell therapy has been approved for R/R DLBCL after 2 prior lines of therapy based on data from single-arm phase 2 trials, with complete responses (CRs) in 40% to 60% of patients. However, a direct comparison with other treatments is not available and, moreover, its true efficacy in real-world patients is unknown. In this single center, retrospective, observational study of 215 patients, we compared outcomes in patients treated with CAR T-cell therapy (n = 69) with a historical population treated with alternate therapies (n = 146). Patients treated with CAR T cell vs alternate therapies demonstrated a CR rate of 52% vs 22% (P < .001), median progression-free survival (PFS) of 5.2 vs 2.3 months (P = .01), and median overall survival (OS) of 19.3 vs 6.5 months (P = .006), and this advantage appeared to persist irrespective of the number of lines of prior therapy. After adjusting for unfavorable pretreatment disease characteristics, superior overall response rate in the CAR T cohort remained significant; however, differences in PFS and OS between cohorts did not. In addition, patients who responded to alternate therapies demonstrated prolonged remissions comparable to those who responded to CAR T therapy. We contend that in select clinical scenarios alternate therapies may be as efficacious as CAR T therapy; thus, additional study is warranted, ideally with randomized prospective trials.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.B. received grant funding from Janssen, Novartis, Epizyme, Xynomics, Bayer, BMS; served as a consultant for Life Sci, GLG, Celgene, Seattle Genetics, Xynomics; and holds honorarium from Dava Oncology. C.H. received honorarium from Invivoscribe Inc. G.S. received research funding from Janssen and Amgen. M.-A.P. reports honoraria from AbbVie, Bellicum, Celgene, Bristol-Myers Squibb, Incyte, Kite/Gilead, Merck, Novartis, Nektar Therapeutics, Omeros, and Takeda; serves on DSMBs for Cidara Therapeutics, Servier and Medigene and the scientific advisory boards of MolMed and NexImmune; received research support for clinical trials from Incyte, Kite/Gilead and Miltenyi Biotec; and serves in a volunteer capacity as a member of the Board of Directors of American Society for Transplantation and Cellular Therapy (ASTCT) and Be The Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Cellular Immunotherapy Data Resource (CIDR) Committee. M.S. has served as a consultant for McKinsey & Company, Angiocrine Bioscience, Inc., Omeros Corporation; received research funding from Angiocrine Bioscience, Inc., and has served on an ad hoc advisory board for Kite–A Gilead Company. P.D. serves on the advisory board for Kite/Gilead. P.H. receives research support from Portola, Novartis/GSK, Molecular Templates, and Janssen Pharmaceuticals and served as consultant for Karyopharm, Juno, Portola, Celgene, and AstraZeneca. S.H. received research funding from ADCT therapeutics, Aileron, Forty-Seven, Verastem, Kyowa Hakko Kirin, Millennium Pharmaceuticals Inc, Celgene, Trillium, and Daiichii Sankyo and consults for Astex, Affimed, Merck Sharp and Dome, Kyowa Hakko Kirin Pharma, Corvus Pharmaceuticals Inc., Celgene, Portola Pharmaceuticals, Takeda Millennium, Innate Pharma, Verastem, Miragen Therapeutics Inc, Seattle Genetics, and ADCT. A.K. receives research funding from AbbVie Pharmaceuticals, Adaptive Biotechnologies, Pharmacyclics, and Seattle Genetics and serves on advisory board for Celgene and Astra Zeneca. A.N. receives honoraria from Janssen, Pharmacyclics, and Prime Oncology; consults for Medscape; serves on advisory board for Janssen; serves on the speakers bureau for Prime Oncology; and receives research funding for Rafael Pharma and Pharmacyclics. A.M. receives research support from Seattle Genetics, Merck, Bristol-Myers Squibb, and Incyte and receives honorarium from Kyowa Hakko Kirin Pharma, Miragen Therapeutics, Takeda Pharmaceuticals, ADC Therapeutics, Seattle Genetics, Cell Medica, Bristol-Myers Squibb, and Erytech Pharma. D. Straus consults for InPractice Elselvier and Seattle Genetics and is on speakers bureau for Medical Crossfire. A.Z. consults for Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnology, and Verastem; serves on advisory boards for MorphoSys, Gilead, Genentech, AbbVie, AstraZeneca, and Pharmacyclics; and receives research funding from MEI Pharmaceuticals, Roche, Gilead, and Beigene. A.Y. receives research support from Janssen, Curis, Merck, BMS, Syndax, and Roche and honorarium from Janssen, AbbVie, Merck, Curis, Epizyme, Roche, and Takeda and consults for Biopath, Xynomics, Epizyme, Roche, Celgene, and HCM. C.S. receives research support from Juno Therapeutics, Celgene, Precision Biosciences, and Sanofi-Genzyme and consults for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite, Celgene, Gamida Cell, and GSK. The remaining authors declare no competing financial interests.
Figures
References
-
- Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30(36):4462-4469. - PMC - PubMed

