Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial
- PMID: 33002429
- PMCID: PMC7616947
- DOI: 10.1016/S0140-6736(20)31553-1
Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial
Abstract
Background: The optimal timing of radiotherapy after radical prostatectomy for prostate cancer is uncertain. We aimed to compare the efficacy and safety of adjuvant radiotherapy versus an observation policy with salvage radiotherapy for prostate-specific antigen (PSA) biochemical progression.
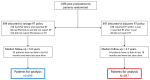
Methods: We did a randomised controlled trial enrolling patients with at least one risk factor (pathological T-stage 3 or 4, Gleason score of 7-10, positive margins, or preoperative PSA ≥10 ng/mL) for biochemical progression after radical prostatectomy (RADICALS-RT). The study took place in trial-accredited centres in Canada, Denmark, Ireland, and the UK. Patients were randomly assigned in a 1:1 ratio to adjuvant radiotherapy or an observation policy with salvage radiotherapy for PSA biochemical progression (PSA ≥0·1 ng/mL or three consecutive rises). Masking was not deemed feasible. Stratification factors were Gleason score, margin status, planned radiotherapy schedule (52·5 Gy in 20 fractions or 66 Gy in 33 fractions), and centre. The primary outcome measure was freedom from distant metastases, designed with 80% power to detect an improvement from 90% with salvage radiotherapy (control) to 95% at 10 years with adjuvant radiotherapy. We report on biochemical progression-free survival, freedom from non-protocol hormone therapy, safety, and patient-reported outcomes. Standard survival analysis methods were used. A hazard ratio (HR) of less than 1 favoured adjuvant radiotherapy. This study is registered with ClinicalTrials.gov, NCT00541047.
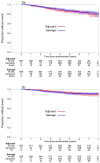
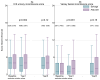
Findings: Between Nov 22, 2007, and Dec 30, 2016, 1396 patients were randomly assigned, 699 (50%) to salvage radiotherapy and 697 (50%) to adjuvant radiotherapy. Allocated groups were balanced with a median age of 65 years (IQR 60-68). Median follow-up was 4·9 years (IQR 3·0-6·1). 649 (93%) of 697 participants in the adjuvant radiotherapy group reported radiotherapy within 6 months; 228 (33%) of 699 in the salvage radiotherapy group reported radiotherapy within 8 years after randomisation. With 169 events, 5-year biochemical progression-free survival was 85% for those in the adjuvant radiotherapy group and 88% for those in the salvage radiotherapy group (HR 1·10, 95% CI 0·81-1·49; p=0·56). Freedom from non-protocol hormone therapy at 5 years was 93% for those in the adjuvant radiotherapy group versus 92% for those in the salvage radiotherapy group (HR 0·88, 95% CI 0·58-1·33; p=0·53). Self-reported urinary incontinence was worse at 1 year for those in the adjuvant radiotherapy group (mean score 4·8 vs 4·0; p=0·0023). Grade 3-4 urethral stricture within 2 years was reported in 6% of individuals in the adjuvant radiotherapy group versus 4% in the salvage radiotherapy group (p=0·020).
Interpretation: These initial results do not support routine administration of adjuvant radiotherapy after radical prostatectomy. Adjuvant radiotherapy increases the risk of urinary morbidity. An observation policy with salvage radiotherapy for PSA biochemical progression should be the current standard after radical prostatectomy.
Funding: Cancer Research UK, MRC Clinical Trials Unit, and Canadian Cancer Society.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing Interests
All authors have completed the ICMJE uniform disclosure form at
Declaration Of Interests
Prof. Parker reports grants, personal fees and other from Bayer, other from AAA and personal fees from Janssen, outside the submitted work.
Prof. Clarke reports personal fees from Janssen, during the conduct of the study; personal fees from Janssen, outside the submitted work.
Dr. Catton reports grants from Canadian Cancer Trials Group, during the conduct of the study; personal fees from Bayer Corp, grants from Astra-Zeneca, personal fees from Abbvie Corp, personal fees from Janssen Corp, personal fees from Astellas Corp, outside the submitted work.
Dr. Payne reports personal fees from Janssen, personal fees from Astellas, personal fees from Astra Zeneca, personal fees from Ferring, personal fees from Ipsen, outside the submitted work.
Dr. Saad reports grants, personal fees and non-financial support from Astellas, grants, personal fees and non-financial support from Amgen, grants, personal fees and non-financial support from Janssen, grants, personal fees and non-financial support from Bayer, grants, personal fees and non-financial support from Sanofi, grants, personal fees and non-financial support from Pfizer, grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from Myovant, outside the submitted work.
Dr. Lindberg reports personal fees and non-financial support from Astellas Phama, personal fees and non-financial support from Bayer, personal fees and non-financial support from Janssen, personal fees and non-financial support from Sanofi Aventis, personal fees from Roche, outside the submitted work.
Dr. Zarkar reports other fees from Bayer, personal fees from Pfizer, personal fees from Janssen, personal fees from Astellas, grants from Sanofi, personal fees from EUSA Pharma, outside the submitted work.
Prof. Parmar reports grants and non-financial support from Astellas, grants and non-financial support from Clovis Oncology, grants and non-financial support from Novartis, grants and non-financial support from Pfizer, grants and non-financial support from Sanofi, outside the submitted work.
Prof. Sydes reports grants and non-financial support from Astellas, grants and non-financial support from Clovis Oncology, grants and non-financial support from Novartis, grants and non-financial support from Pfizer, personal fees from Eli Lilly, grants, personal fees and non-financial support from Janssen, grants and non-financial support from Sanofi, outside the submitted work.
All remaining authors declare none.
Figures
Comment in
-
Timing of radiotherapy after radical prostatectomy.Lancet. 2020 Oct 31;396(10260):1374-1375. doi: 10.1016/S0140-6736(20)31957-7. Epub 2020 Sep 28. Lancet. 2020. PMID: 33002430 No abstract available.
-
Salvage radiotherapy: a new standard of care.Nat Rev Clin Oncol. 2021 Jan;18(1):5. doi: 10.1038/s41571-020-00443-3. Nat Rev Clin Oncol. 2021. PMID: 33067593 No abstract available.
-
Salvage radiotherapy: a new standard of care.Nat Rev Urol. 2020 Dec;17(12):657. doi: 10.1038/s41585-020-00392-7. Nat Rev Urol. 2020. PMID: 33077912 No abstract available.
-
Adjuvant Versus Early Salvage Radiation Therapy After Radical Prostatectomy for Men With Adverse Pathologic Features-The Debate Continues.Int J Radiat Oncol Biol Phys. 2021 Mar 15;109(4):839-843. doi: 10.1016/j.ijrobp.2020.12.022. Int J Radiat Oncol Biol Phys. 2021. PMID: 33610298 No abstract available.
-
Re: Timing of Radiotherapy After Radical Prostatectomy (RadicalS-RT): A Randomised, Controlled Phase 3 Trial.Eur Urol. 2021 Jul;80(1):117. doi: 10.1016/j.eururo.2021.02.016. Epub 2021 Feb 19. Eur Urol. 2021. PMID: 33612373 No abstract available.
-
Postoperative radiotherapy in prostate cancer.Lancet. 2021 May 1;397(10285):1623. doi: 10.1016/S0140-6736(21)00273-7. Lancet. 2021. PMID: 33933203 No abstract available.
-
Radikale Prostatektomie: Adjuvante versus Salvage-Radiatio.Aktuelle Urol. 2022 Feb;53(1):12-13. doi: 10.1055/a-1559-4803. Epub 2022 Jan 25. Aktuelle Urol. 2022. PMID: 35078254 German. No abstract available.
-
Immediate or salvage radiotherapy after radical prostatectomy: Do we finally know?Natl Med J India. 2021 Sep-Oct;34(5):282-284. doi: 10.25259/NMJI_127_21. Natl Med J India. 2021. PMID: 35593236 No abstract available.
References
-
- Vatne K, Stensvold A, Myklebust TA, Moller B, Svindland A, Kvale R, et al. Pre- and post-prostatectomy variables associated with pelvic post-operative radiotherapy in prostate cancer patients: a national registry-based study. Acta Oncol. 2017;56(10):1295–301. - PubMed
-
- Parry MG, Sujenthiran A, Cowling TE, Nossiter J, Cathcart P, Clarke NW, et al. Impact of cancer service centralisation on the radical treatment of men with high-risk and locally advanced prostate cancer: A national cross-sectional analysis in England. Int J Cancer. 2019;145(1):40–8. doi: 10.1002/ijc.32068. - DOI - PMC - PubMed
-
- Swanson GP, Goldman B, Tangen CM, Chin J, Messing E, Canby-Hagino E, et al. The prognostic impact of seminal vesicle involvement found at prostatectomy and the effects of adjuvant radiation: data from Southwest Oncology Group 8794. J Urol. 2008;180(6):2453–7. doi: 10.1016/j.juro.2008.08.037. discussion 8. - DOI - PMC - PubMed
-
- Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) The Lancet. 2012;380(9858):2018–27. - PubMed
-
- Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366(9485):572–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous