Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data
- PMID: 33002431
- PMCID: PMC7611137
- DOI: 10.1016/S0140-6736(20)31952-8
Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data
Abstract
Background: It is unclear whether adjuvant or early salvage radiotherapy following radical prostatectomy is more appropriate for men who present with localised or locally advanced prostate cancer. We aimed to prospectively plan a systematic review of randomised controlled trials (RCTs) comparing these radiotherapy approaches.
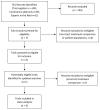
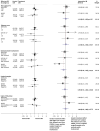
Methods: We used a prospective framework for adaptive meta-analysis (FAME), starting the review process while eligible trials were ongoing. RCTs were eligible if they aimed to compare immediate adjuvant radiotherapy versus early salvage radiotherapy, following radical prostatectomy in men (age ≥18 years) with intermediate-risk or high-risk, localised or locally advanced prostate cancer. We searched trial registers and conference proceedings until July 8, 2020, to identify eligible RCTs. By establishing the ARTISTIC collaboration with relevant trialists, we were able to anticipate when eligible trial results would emerge, and we developed and registered a protocol with PROSPERO before knowledge of the trial results (CRD42019132669). We used a harmonised definition of event-free survival, as the time from randomisation until the first evidence of either biochemical progression (prostate-specific antigen [PSA] ≥0·4 ng/mL and rising after completion of any postoperative radiotherapy), clinical or radiological progression, initiation of a non-trial treatment, death from prostate cancer, or a PSA level of at least 2·0 ng/mL at any time after randomisation. We predicted when we would have sufficient power to assess whether adjuvant radiotherapy was superior to early salvage radiotherapy. Investigators supplied results for event-free survival, both overall and within predefined patient subgroups. Hazard ratios (HRs) for the effects of radiotherapy timing on event-free survival and subgroup interactions were combined using fixed-effect meta-analysis.
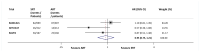
Findings: We identified three eligible trials and were able to obtain updated results for event-free survival for 2153 patients recruited between November, 2007, and December, 2016. Median follow-up ranged from 60 months to 78 months, with a maximum follow-up of 132 months. 1075 patients were randomly assigned to receive adjuvant radiotherapy and 1078 to a policy of early salvage radiotherapy, of whom 421 (39·1%) had commenced treatment at the time of analysis. Patient characteristics were balanced within trials and overall. Median age was similar between trials at 64 or 65 years (with IQRs ranging from 59 to 68 years) across the three trials and most patients (1671 [77·6%]) had a Gleason score of 7. All trials were assessed as having low risk of bias. Based on 270 events, the meta-analysis showed no evidence that event-free survival was improved with adjuvant radiotherapy compared with early salvage radiotherapy (HR 0·95, 95% CI 0·75-1·21; p=0·70), with only a 1 percentage point (95% CI -2 to 3) change in 5-year event-free survival (89% vs 88%). Results were consistent across trials (heterogeneity p=0·18; I2=42%).
Interpretation: This collaborative and prospectively designed systematic review and meta-analysis suggests that adjuvant radiotherapy does not improve event-free survival in men with localised or locally advanced prostate cancer. Until data on long-term outcomes are available, early salvage treatment would seem the preferable treatment policy as it offers the opportunity to spare many men radiotherapy and its associated side-effects.
Funding: UK Medical Research Council.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CV, DF, AK, MP, PR, AC, CB, MB, SC, SF, CBr and JT report no financial conflicts of interest in relation to this work. CP reports grants received from Bayer; personal fees received from Bayer and Janssen and other (including speaker fees, advisory board membership and honoraria) form Bayer, AAA and Janssen, outside the submitted work.
PS reports honoraria, speaker fees and advisory board fees from Ipsen, Astellas, Bouchara, Takeda and Ferring, during the conduct of the study; as well as other relationships and activities from Janssen, Bayer and Sanofi, all outside the submitted work.
CFB reports grants from New Zealand Health Research Council, Australian National Health and Medical Research Council, Auckland Hospital Charitable Trust, TROG Seed Funding, and Genesis Oncology Trust during the conduct of the study.
MS reports grants and non-financial support from Astellas, Clovis Oncology, Janssen, Novartis, Pfizer and Sanofi; and personal fees from Eli Lilly and Janssen, outside the submitted work. IL reports other financial relationships from Sanofi, Ipsen and Astellas, outside the submitted work.
MKP reports grants and non-financial support from Astellas, Clovis Oncology, Novartis, Pfizer, and Sanofi, outside the submitted work.
Figures
Comment in
-
Timing of radiotherapy after radical prostatectomy.Lancet. 2020 Oct 31;396(10260):1374-1375. doi: 10.1016/S0140-6736(20)31957-7. Epub 2020 Sep 28. Lancet. 2020. PMID: 33002430 No abstract available.
-
Adjuvant Versus Early Salvage Radiation Therapy After Radical Prostatectomy for Men With Adverse Pathologic Features-The Debate Continues.Int J Radiat Oncol Biol Phys. 2021 Mar 15;109(4):839-843. doi: 10.1016/j.ijrobp.2020.12.022. Int J Radiat Oncol Biol Phys. 2021. PMID: 33610298 No abstract available.
-
Immediate or salvage radiotherapy after radical prostatectomy: Do we finally know?Natl Med J India. 2021 Sep-Oct;34(5):282-284. doi: 10.25259/NMJI_127_21. Natl Med J India. 2021. PMID: 35593236 No abstract available.
References
-
- Bolla M, Poppel H, Collette L, van Cangh P, Vekemans K, da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) The Lancet. 2005;366:572–8. - PubMed
-
- Wiegel T, Bottke D, Steiner U, Siegmann A, Golz R, Storkel S, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30. - PubMed
-
- Sineshaw HM, Gray PJ, Efstathiou JA, Jemal A. Declining Use of Radiotherapy for Adverse Features After RadicalProstatectomy: Results From the National Cancer Data Base. Eur Urol. 2015;68:768–74. - PubMed
-
- Parker C, Sydes MR, Catton C, Kynaston H, Logue J, Murphy C, et al. Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int. 2007;99(6):1376–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

