Effect of alanine supplementation on oxalate synthesis
- PMID: 33002578
- PMCID: PMC7722081
- DOI: 10.1016/j.bbadis.2020.165981
Effect of alanine supplementation on oxalate synthesis
Abstract
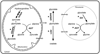
The Primary Hyperoxalurias (PH) are rare disorders of metabolism leading to excessive endogenous synthesis of oxalate and recurring calcium oxalate kidney stones. Alanine glyoxylate aminotransferase (AGT), deficient in PH type 1, is a key enzyme in limiting glyoxylate oxidation to oxalate. The affinity of AGT for its co-substrate, alanine, is low suggesting that its metabolic activity could be sub-optimal in vivo. To test this hypothesis, we examined the effect of L-alanine supplementation on oxalate synthesis in cell culture and in mouse models of Primary Hyperoxaluria Type 1 (Agxt KO), Type 2 (Grhpr KO) and in wild-type mice. Our results demonstrated that increasing L-alanine in cells decreased synthesis of oxalate and increased viability of cells expressing GO and AGT when incubated with glycolate. In both wild type and Grhpr KO male and female mice, supplementation with 10% dietary L-alanine significantly decreased urinary oxalate excretion ~30% compared to baseline levels. This study demonstrates that increasing the availability of L-alanine can increase the metabolic efficiency of AGT and reduce oxalate synthesis.
Keywords: Alanine; Alanine:glyoxylate aminotransferase; Kidney stones; Oxalate; Primary hyperoxaluria.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interest
The authors declare that they have no conflict of interest with the content of the article.
KW is consultant for Synlogic Therapeutics, Oxidien Therapeutics, Novome Therapeutics, and BioBridge Therapeutics. JK is a consultant for Synlogic, Novome and Chinook Therapeutics. The other authors have no disclosures.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Comment in
-
Urolithiasis/Endourology.J Urol. 2021 Apr;205(4):1219-1220. doi: 10.1097/JU.0000000000001604. Epub 2021 Jan 20. J Urol. 2021. PMID: 33467893 No abstract available.
Similar articles
-
Effects of alanine:glyoxylate aminotransferase variants and pyridoxine sensitivity on oxalate metabolism in a cell-based cytotoxicity assay.Biochim Biophys Acta. 2016 Jun;1862(6):1055-62. doi: 10.1016/j.bbadis.2016.02.004. Epub 2016 Feb 6. Biochim Biophys Acta. 2016. PMID: 26854734 Free PMC article.
-
Metabolism of (13)C5-hydroxyproline in mouse models of Primary Hyperoxaluria and its inhibition by RNAi therapeutics targeting liver glycolate oxidase and hydroxyproline dehydrogenase.Biochim Biophys Acta. 2016 Feb;1862(2):233-9. doi: 10.1016/j.bbadis.2015.12.001. Epub 2015 Dec 2. Biochim Biophys Acta. 2016. PMID: 26655602 Free PMC article.
-
Hydroxyproline metabolism in mouse models of primary hyperoxaluria.Am J Physiol Renal Physiol. 2012 Mar 15;302(6):F688-93. doi: 10.1152/ajprenal.00473.2011. Epub 2011 Dec 21. Am J Physiol Renal Physiol. 2012. PMID: 22189945 Free PMC article.
-
Human glyoxylate metabolism revisited: New insights pointing to multi-organ involvement with implications for siRNA-based therapies in primary hyperoxaluria.J Inherit Metab Dis. 2025 Jan;48(1):e12817. doi: 10.1002/jimd.12817. Epub 2024 Nov 24. J Inherit Metab Dis. 2025. PMID: 39582099 Free PMC article. Review.
-
[From gene to disease; primary hyperoxaluria type I caused by mutations in the AGXT gene].Ned Tijdschr Geneeskd. 2006 Jul 29;150(30):1669-72. Ned Tijdschr Geneeskd. 2006. PMID: 16922352 Review. Dutch.
Cited by
-
Repeated administration of L-alanine to mice reduces behavioural despair and increases hippocampal mammalian target of rapamycin signalling: Analysis of gender and metabolic effects.J Psychopharmacol. 2025 Jun;39(6):612-623. doi: 10.1177/02698811251332838. Epub 2025 Apr 17. J Psychopharmacol. 2025. PMID: 40242990 Free PMC article.
-
The gut-joint axis mediates the TNF-induced RA process and PBMT therapeutic effects through the metabolites of gut microbiota.Gut Microbes. 2023 Dec;15(2):2281382. doi: 10.1080/19490976.2023.2281382. Epub 2023 Nov 28. Gut Microbes. 2023. PMID: 38017660 Free PMC article.
-
Biomedical applications of L-alanine produced by Pediococcus acidilactici BD16 (alaD+).Appl Microbiol Biotechnol. 2022 Feb;106(4):1435-1446. doi: 10.1007/s00253-022-11766-9. Epub 2022 Jan 28. Appl Microbiol Biotechnol. 2022. PMID: 35089399
-
Updated Gene Therapy for Renal Inborn Errors of Metabolism.Genes (Basel). 2025 Apr 29;16(5):516. doi: 10.3390/genes16050516. Genes (Basel). 2025. PMID: 40428338 Free PMC article. Review.
References
-
- Danpure CJ, Primary Hyperoxaluria, in: B.A. Scriver CR (Ed.), The Metabolic and Molecular Bases of Inherited Disease, McGraw-Hill, New-York, 2001: pp. 3323–3367.
-
- Hoppe B, Beck BB, Milliner DS, The primary hyperoxalurias, Kidney Int 75 (2009) 1264–71. https://doi.org/ki200932 [pii] 10.1038/ki.2009.32. - DOI - PMC - PubMed
-
- Danpure CJ, Jennings PR, Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I, FEBS Lett 201 (1986) 20–24. - PubMed
-
- Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP, The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II, Hum. Mol. Genet 8 (1999) 2063–2069. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

