Activation of Mechanistic Target of Rapamycin (mTOR) in Human Endothelial Cells Infected with Pathogenic Spotted Fever Group Rickettsiae
- PMID: 33003310
- PMCID: PMC7582468
- DOI: 10.3390/ijms21197179
Activation of Mechanistic Target of Rapamycin (mTOR) in Human Endothelial Cells Infected with Pathogenic Spotted Fever Group Rickettsiae
Abstract
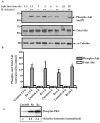
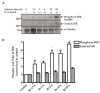
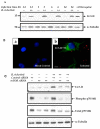
Attributed to the tropism for host microvascular endothelium lining the blood vessels, vascular inflammation and dysfunction represent salient features of rickettsial pathogenesis, yet the details of fundamentally important pathogen interactions with host endothelial cells (ECs) as the primary targets of infection remain poorly appreciated. Mechanistic target of rapamycin (mTOR), a serine/threonine protein kinase of the phosphatidylinositol kinase-related kinase family, assembles into two functionally distinct complexes, namely mTORC1 (Raptor) and mTORC2 (Rictor), implicated in the determination of innate immune responses to intracellular pathogens via transcriptional regulation. In the present study, we investigated activation status of mTOR and its potential contributions to host EC responses during Rickettsia rickettsii and R. conorii infection. Protein lysates from infected ECs were analyzed for threonine 421/serine 424 phosphorylation of p70 S6 kinase (p70 S6K) and that of serine 2448 on mTOR itself as established markers of mTORC1 activation. For mTORC2, we assessed phosphorylation of protein kinase B (PKB or Akt) and protein kinase C (PKC), respectively, on serine 473 and serine 657. The results suggest increased phosphorylation of p70 S6K and mTOR during Rickettsia infection of ECs as early as 3 h and persisting for up to 24 h post-infection. The steady-state levels of phospho-Akt and phospho-PKC were also increased. Infection with pathogenic rickettsiae also resulted in the formation of microtubule-associated protein 1A/1B-light chain 3 (LC3-II) puncta and increased lipidation of LC3-II, a response significantly inhibited by introduction of siRNA targeting mTORC1 into ECs. These findings thus yield first evidence for the activation of both mTORC1 and mTORC2 during EC infection in vitro with Rickettsia species and suggest that early induction of autophagy in response to intracellular infection might be regulated by this important pathway known to function as a central integrator of cellular immunity and inflammation.
Keywords: Akt (protein kinase B); Rickettsia; endothelial cells; mTOR; protein kinase C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sahni A., Narra H.P., Walker D.H., Sahni S.K. Endothelial activation and injury: The mechanisms of rickettsial vasculitis. In: Gavins F., Stokes K.Y., editors. Vascular Responses to Pathogens. Elsevier Press; Atlanta, GA, USA: 2016. pp. 111–122.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

