Bioactive Ent-Kaurane Diterpenes Oridonin and Irudonin Prevent Cancer Cells Migration by Interacting with the Actin Cytoskeleton Controller Ezrin
- PMID: 33003361
- PMCID: PMC7582544
- DOI: 10.3390/ijms21197186
Bioactive Ent-Kaurane Diterpenes Oridonin and Irudonin Prevent Cancer Cells Migration by Interacting with the Actin Cytoskeleton Controller Ezrin
Abstract
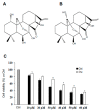
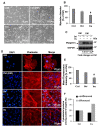
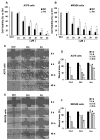
The ent-kaurane diterpene oridonin was reported to inhibit cell migration and invasion in several experimental models. However, the process by which this molecule exerts its anti-metastatic action has not been yet elucidated. In this article, we have investigated the anti-metastatic activity of Oridonin and of one homolog, Irudonin, with the aim to shed light on the molecular mechanisms underlying the biological activity of these ent-kaurane diterpenes. Cell-based experiments revealed that both compounds are able to affect differentiation and cytoskeleton organization in mouse differentiating myoblasts, but also to impair migration, invasion and colony formation ability of two different metastatic cell lines. Using a compound-centric proteomic approach, we identified some potential targets of the two bioactive compounds among cytoskeletal proteins. Among them, Ezrin, a protein involved in the actin cytoskeleton organization, was further investigated. Our results confirmed the pivotal role of Ezrin in regulating cell migration and invasion, and indicate this protein as a potential target for new anti-cancer therapeutic approaches. The interesting activity profile, the good selectivity towards cancer cells, and the lower toxicity with respect to Oridonin, all suggest that Irudonin is a very promising anti-metastatic agent.
Keywords: 9 activity; cancer metastasis; cell Invasion; cell migration; ent-kaurane diterpenes; ezrin; matrix metalloproteinase (MMP)-2; proteomics; target identification.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

