Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993-Mid 2020: A Comprehensive Analysis
- PMID: 33003400
- PMCID: PMC7600114
- DOI: 10.3390/medicines7100062
Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993-Mid 2020: A Comprehensive Analysis
Abstract
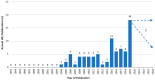
Background: A large number of idiosyncratic drug induced liver injury (iDILI) and herb induced liver injury(HILI) cases of variable quality has been published but some are a matter of concern if the cases were not evaluated for causality using a robust causality assessment method (CAM) such as RUCAM (Roussel Uclaf Causality Assessment Method) as diagnostiinjuryc algorithm. The purpose of this analysis was to evaluate the worldwide use of RUCAM in iDILI and HILI cases. Methods: The PubMed database (1993-30 June 2020) was searched for articles by using the following key terms: Roussel Uclaf Causality Assessment Method; RUCAM; Idiosyncratic drug induced liver injury; iDILI; Herb induced liver injury; HILI. Results: Considering reports published worldwide since 1993, our analysis showed the use of RUCAM for causality assessment in 95,885 cases of liver injury including 81,856 cases of idiosyncratic DILI and 14,029 cases of HILI. Among the top countries providing RUCAM based DILI cases were, in decreasing order, China, the US, Germany, Korea, and Italy, with China, Korea, Germany, India, and the US as the top countries for HILI. Conclusion: Since 1993 RUCAM is certainly the most widely used method to assess causality in IDILI and HILI. This should encourage practitioner, experts, and regulatory agencies to use it in order to reinforce their diagnosis and to take sound decisions.
Keywords: DILI; HILI; RUCAM; Roussel Uclaf Causality Assessment Method; diagnostic algorithm; herb induced liver injury; iDILI; iDrug induced liver injury.
Conflict of interest statement
The authors declared that they have no conflict of interests regarding this invited article.
Figures



References
-
- Uetrecht J. Mechanistic studies of idiosyncratic DILI: Clinical implications. Front. Pharmacol. 2019;10:837. doi: 10.3389/fphar.2019.00837. In: Special issue: Clinical drug induced liver injury: Current diagnostic and mechanistic challenges, guest editors: Rolf Teschke, Gaby Danan & James H. Lewis. - DOI - PMC - PubMed
-
- Chen M., Will Y. In: Drug-Induced Liver Toxicity. James K., David C., editors. Springer Protocols, Springer Nature; Berlin, Germany: 2018. (Series: Methods in Pharmacology and Toxicology).
-
- Teschke R., Danan G. Causality assessment methods in drug-induced liver injury. In: James K., David C.C., Minjun C., Yvonne W., editors. Drug-Induced Liver Toxicity. Springer Nature; Berlin, Germany: 2018. pp. 555–594. (Series: Methods in Pharmacology and Toxicology/Y). Chapter 27. - DOI
Publication types
LinkOut - more resources
Full Text Sources

