Systemic Treatment for Advanced and Metastatic Malignant Peripheral Nerve Sheath Tumors-A Sarcoma Reference Center Experience
- PMID: 33003503
- PMCID: PMC7601777
- DOI: 10.3390/jcm9103157
Systemic Treatment for Advanced and Metastatic Malignant Peripheral Nerve Sheath Tumors-A Sarcoma Reference Center Experience
Abstract
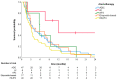
Malignant peripheral nerve sheath tumor (MPNST) is a rare type of soft tissue sarcomas. The localized disease is usually treated with surgery along with perioperative chemo- or radiotherapy. However, up to 70% of patients can develop distant metastases. The study aimed to evaluate the modes and outcomes of systemic treatment of patients with diagnosed MPNST treated in a reference center. In total, 115 patients (56 female and 59 male) diagnosed with MPNST and treated due to unresectable or metastatic disease during 2000-2019 were included in the retrospective analysis. Schemes of systemic therapy and the outcomes-progression-free survival (PFS) and overall survival (OS)-were evaluated. The median PFS in the first line was 3.9 months (95% CI 2.5-5.4). Doxorubicin-based regimens were the most commonly used in the first line (50.4% of patients). There were no significant differences in PFS between chemotherapy regimens most commonly used in the first line (p = 0.111). The median OS was 15.0 months (95% CI 11.0-19.0) and the one-year OS rate was 63%. MPNST are resistant to the majority of systemic therapies, resulting in poor survival in advanced settings. Chemotherapy with doxorubicin and ifosfamide is associated with the best response and longest PFS. Future studies and the development of novel treatment options are necessary for the improvement of treatment outcomes.
Keywords: MPNST; chemotherapy; doxorubicin; ifosfamide; pazopanib; sarcoma.
Conflict of interest statement
P.S. has received travel grants from MSD, Roche, and Pierre Fabre. P.T. has received honoraria and travel grants from Roche, Eli Lilly, Bayer, and Novartis. A.M.C. has received travel grants, payment for lectures, and consulting fees from BMS, MSD, Roche, and Novartis. P.R. has received honoraria for lectures from Novartis, Roche, Pfizer, BMS, Eli Lilly, and MSD and served as a member of the advisory board for Novartis, Merck, Amgen, Blueprint Medicine, Roche, BMS, and MSD. T.Ś. has received travel grants, payment for lectures, and consulting fees from BMS, MSD, Roche, and Novartis. H.K.P. has received honoraria and travel grants from MSD, Novartis, BMS, and Roche. K.K. has received honoraria and travel grants from BMS, MSD, Roche, and Novartis. S.F. declares no conflict of interest.
Figures



Similar articles
-
First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study.Ann Oncol. 2011 Jan;22(1):207-214. doi: 10.1093/annonc/mdq338. Epub 2010 Jul 23. Ann Oncol. 2011. PMID: 20656792 Free PMC article.
-
Malignant peripheral nerve sheath tumors - Outcomes and prognostic factors based on the reference center experience.Surg Oncol. 2020 Dec;35:276-284. doi: 10.1016/j.suronc.2020.09.011. Epub 2020 Sep 12. Surg Oncol. 2020. PMID: 32949967
-
Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy.Am J Clin Oncol. 2011 Aug;34(4):417-21. doi: 10.1097/COC.0b013e3181e9c08a. Am J Clin Oncol. 2011. PMID: 20838322
-
Systemic Options for Malignant Peripheral Nerve Sheath Tumors.Curr Treat Options Oncol. 2021 Feb 27;22(4):33. doi: 10.1007/s11864-021-00830-7. Curr Treat Options Oncol. 2021. PMID: 33641042 Review.
-
Non-cytotoxic systemic treatment in malignant peripheral nerve sheath tumors (MPNST): A systematic review from bench to bedside.Crit Rev Oncol Hematol. 2019 Jun;138:223-232. doi: 10.1016/j.critrevonc.2019.04.007. Epub 2019 Apr 19. Crit Rev Oncol Hematol. 2019. PMID: 31092379
Cited by
-
Pharmacogenomic synthetic lethal screens reveal hidden vulnerabilities and new therapeutic approaches for treatment of NF1-associated tumors.bioRxiv [Preprint]. 2024 Nov 1:2024.03.25.585959. doi: 10.1101/2024.03.25.585959. bioRxiv. 2024. PMID: 38585724 Free PMC article. Preprint.
-
Metastatic malignant peripheral nerve sheath tumour in a patient with neurofibromatosis 1 and review of contemporary systemic treatments.BMJ Case Rep. 2022 Oct 3;15(10):e250462. doi: 10.1136/bcr-2022-250462. BMJ Case Rep. 2022. PMID: 36192032 Free PMC article.
-
Targeting the Galectin-1/Ras Interaction for Treating Malignant Peripheral Nerve Sheath Tumors.Res Sq [Preprint]. 2024 Oct 16:rs.3.rs-5263500. doi: 10.21203/rs.3.rs-5263500/v1. Res Sq. 2024. PMID: 39483902 Free PMC article. Preprint.
-
Brain metastases of sarcoma: a rare phenomenon in rare tumours.J Cancer Res Clin Oncol. 2023 Dec;149(20):18271-18281. doi: 10.1007/s00432-023-05451-1. Epub 2023 Nov 23. J Cancer Res Clin Oncol. 2023. PMID: 37994983 Free PMC article. Review.
-
The quest for effective immunotherapies against malignant peripheral nerve sheath tumors: Is there hope?Mol Ther Oncolytics. 2023 Jul 31;30:227-237. doi: 10.1016/j.omto.2023.07.008. eCollection 2023 Sep 21. Mol Ther Oncolytics. 2023. PMID: 37680255 Free PMC article. Review.
References
-
- Fletcher C.D.M. Soft Tissue and Bone Tumours WHO Classification of Tumours. 5th ed. IARC; Lyon, France: 2013.
-
- Hirbe A.C., Gutmann D.H. The management of neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors: Challenges, progress, and future prospects. Expert Opin. Orphan Drugs. 2017;5:623–631. doi: 10.1080/21678707.2017.1348294. - DOI
-
- Czarnecka A.M., Sobczuk P., Zdzienicki M., Spałek M., Rutkowski P. Malignant peripheral nerve sheath tumour (MPNST) Oncol. Clin. Pract. 2019;14:364–376. doi: 10.5603/OCP.2018.0050. - DOI
LinkOut - more resources
Full Text Sources
Medical

