Scaffold-Mediated Gene Delivery for Osteochondral Repair
- PMID: 33003607
- PMCID: PMC7601511
- DOI: 10.3390/pharmaceutics12100930
Scaffold-Mediated Gene Delivery for Osteochondral Repair
Abstract
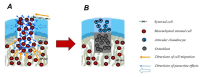
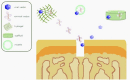
Osteochondral defects involve both the articular cartilage and the underlying subchondral bone. If left untreated, they may lead to osteoarthritis. Advanced biomaterial-guided delivery of gene vectors has recently emerged as an attractive therapeutic concept for osteochondral repair. The goal of this review is to provide an overview of the variety of biomaterials employed as nonviral or viral gene carriers for osteochondral repair approaches both in vitro and in vivo, including hydrogels, solid scaffolds, and hybrid materials. The data show that a site-specific delivery of therapeutic gene vectors in the context of acellular or cellular strategies allows for a spatial and temporal control of osteochondral neotissue composition in vitro. In vivo, implantation of acellular hydrogels loaded with nonviral or viral vectors has been reported to significantly improve osteochondral repair in translational defect models. These advances support the concept of scaffold-mediated gene delivery for osteochondral repair.
Keywords: controlled delivery; gene therapy; osteochondral repair; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

