Automated Annotation of Sphingolipids Including Accurate Identification of Hydroxylation Sites Using MS n Data
- PMID: 33003696
- PMCID: PMC7581017
- DOI: 10.1021/acs.analchem.0c03016
Automated Annotation of Sphingolipids Including Accurate Identification of Hydroxylation Sites Using MS n Data
Abstract
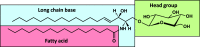
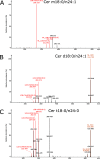
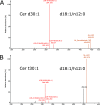
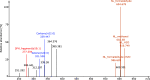
Sphingolipids constitute a heterogeneous lipid category that is involved in many key cellular functions. For high-throughput analyses of sphingolipids, tandem mass spectrometry (MS/MS) is the method of choice, offering sufficient sensitivity, structural information, and quantitative precision for detecting hundreds to thousands of species simultaneously. While glycerolipids and phospholipids are predominantly non-hydroxylated, sphingolipids are typically dihydroxylated. However, species containing one or three hydroxylation sites can be detected frequently. This variability in the number of hydroxylation sites on the sphingolipid long-chain base and the fatty acyl moiety produces many more isobaric species and fragments than for other lipid categories. Due to this complexity, the automated annotation of sphingolipid species is challenging, and incorrect annotations are common. In this study, we present an extension of the Lipid Data Analyzer (LDA) "decision rule set" concept that considers the structural characteristics that are specific for this lipid category. To address the challenges inherent to automated annotation of sphingolipid structures from MS/MS data, we first developed decision rule sets using spectra from authentic standards and then tested the applicability on biological samples including murine brain and human plasma. A benchmark test based on the murine brain samples revealed a highly improved annotation quality as measured by sensitivity and reliability. The results of this benchmark test combined with the easy extensibility of the software to other (sphingo)lipid classes and the capability to detect and correctly annotate novel sphingolipid species make LDA broadly applicable to automated sphingolipid analysis, especially in high-throughput settings.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Levery S. B. Glycosphingolipid Structural Analysis and Glycosphingolipidomics. Methods Enzymol. 2005, 405, 300–369. - PubMed
-
- Itonori S.; Sugita M. In Experimental Glycoscience: Glycochemistry, Taniguchi N.; Suzuki A.; Ito Y.; Narimatsu H.; Kawasaki T.; Hase S., Eds.; Springer: Japan: Tokyo, 2008; pp 74–76.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

