Metal-Dependent Cytotoxic and Kinesin Spindle Protein Inhibitory Activity of Ru, Os, Rh, and Ir Half-Sandwich Complexes of Ispinesib-Derived Ligands
- PMID: 33003697
- PMCID: PMC7584371
- DOI: 10.1021/acs.inorgchem.0c00957
Metal-Dependent Cytotoxic and Kinesin Spindle Protein Inhibitory Activity of Ru, Os, Rh, and Ir Half-Sandwich Complexes of Ispinesib-Derived Ligands
Abstract
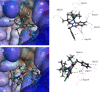
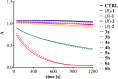
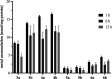
Ispinesib is a potent inhibitor of kinesin spindle protein (KSP), which has been identified as a promising target for antimitotic anticancer drugs. Herein, we report the synthesis of half-sandwich complexes of Ru, Os, Rh, and Ir bearing the ispinesib-derived N,N-bidentate ligands (R)- and (S)-2-(1-amino-2-methylpropyl)-3-benzyl-7-chloroquinazolin-4(3H)-one and studies on their chemical and biological properties. Using the enantiomerically pure (R)- and (S)-forms of the ligand, depending on the organometallic moiety, either the SM,R or RM,S diastereomers, respectively, were observed in the molecular structures of the Ru- and Os(cym) (cym = η6-p-cymene) compounds, whereas the RM,R or SM,S diastereomers were found for the Rh- and Ir(Cp*) (Cp* = η5-pentamethylcyclopentadienyl) derivatives. However, density functional theory (DFT) calculations suggest that the energy difference between the diastereomers is very small, and therefore a mixture of both will be present in solution. The organometallics exhibited varying antiproliferative activity in a series of human cancer cell lines, with the complexes featuring the (R)-enantiomer of the ligand being more potent than the (S)-configured counterparts. Notably, the Rh and Ir complexes demonstrated high KSP inhibitory activity, even at 1 nM concentration, which was independent of the chirality of the ligand, whereas the Ru and especially the Os derivatives were much less active.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
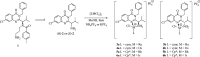
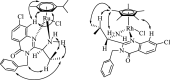



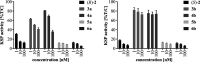

References
-
- Behera M.; Owonikoko T. K.; Kim S.; Chen Z.; Higgins K.; Ramalingam S. S.; Shin D. M.; Khuri F. R.; Beitler J. J.; Saba N. F. Concurrent therapy with taxane versus non-taxane containing regimens in locally advanced squamous cell carcinomas of the head and neck (SCCHN): a systematic review. Oral Oncol. 2014, 50 (9), 888–94. 10.1016/j.oraloncology.2014.06.014. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

