Dual regulation by subcellular calcium heterogeneity and heart rate variability on cardiac electromechanical dynamics
- PMID: 33003911
- PMCID: PMC7502019
- DOI: 10.1063/5.0019313
Dual regulation by subcellular calcium heterogeneity and heart rate variability on cardiac electromechanical dynamics
Abstract
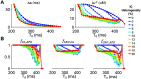
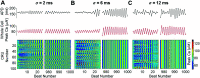
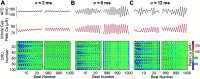
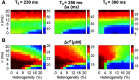
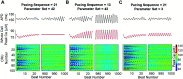
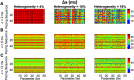
Heart rate constantly varies under physiological conditions, termed heart rate variability (HRV), and in clinical studies, low HRV is associated with a greater risk of cardiac arrhythmias. Prior work has shown that HRV influences the temporal patterns of electrical activity, specifically the formation of pro-arrhythmic alternans, a beat-to-beat alternation in the action potential duration (APD), or intracellular calcium (Ca) levels. We previously showed that HRV may be anti-arrhythmic by disrupting APD and Ca alternations in a homogeneous cardiac myocyte. Here, we expand on our previous work, incorporating variation in subcellular Ca handling (also known to influence alternans) into a nonlinear map model of a cardiac myocyte composed of diffusively coupled Ca release units (CRUs). Ca-related parameters and initial conditions of each CRU are varied to mimic subcellular Ca heterogeneity, and a stochastic pacing sequence reproduces HRV. We find that subcellular Ca heterogeneity promotes the formation of spatially discordant subcellular alternans patterns, which decreases whole cell Ca and APD alternation for low and moderate HRV, while high subcellular Ca heterogeneity and HRV both promote electromechanical desynchronization. Finally, we find that for low and moderate HRV, both the specific subcellular Ca-related parameters and the pacing sequences influence measures of electromechanical dynamics, while for high HRV, these measures depend predominantly on the pacing sequence. Our results suggest that pro-arrhythmic subcellular discordant alternans tend to form for low levels of HRV, while high HRV may be anti-arrhythmic due to mitigated influence from subcellular Ca heterogeneity and desynchronization of APD from Ca instabilities.
Figures







Similar articles
-
Long-term changes in heart rate and electrical remodeling contribute to alternans formation in heart failure: a patient-specific in silico study.Am J Physiol Heart Circ Physiol. 2023 Aug 1;325(2):H414-H431. doi: 10.1152/ajpheart.00220.2023. Epub 2023 Jul 7. Am J Physiol Heart Circ Physiol. 2023. PMID: 37417871 Free PMC article.
-
Heart rate variability alters cardiac repolarization and electromechanical dynamics.J Theor Biol. 2018 Apr 7;442:31-43. doi: 10.1016/j.jtbi.2018.01.007. Epub 2018 Jan 11. J Theor Biol. 2018. PMID: 29337261
-
Dynamical mechanism for subcellular alternans in cardiac myocytes.Circ Res. 2009 Aug 14;105(4):335-42. doi: 10.1161/CIRCRESAHA.109.197590. Epub 2009 Jul 23. Circ Res. 2009. PMID: 19628792 Free PMC article.
-
Cardiac alternans and intracellular calcium cycling.Clin Exp Pharmacol Physiol. 2014 Jul;41(7):524-32. doi: 10.1111/1440-1681.12231. Clin Exp Pharmacol Physiol. 2014. PMID: 25040398 Free PMC article. Review.
-
Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes.J Physiol. 2003 Jan 1;546(Pt 1):19-31. doi: 10.1113/jphysiol.2002.025239. J Physiol. 2003. PMID: 12509476 Free PMC article. Review.
Cited by
-
Electrophysiological Mechanisms Underlying T-Wave Alternans and Their Role in Arrhythmogenesis.Front Physiol. 2021 Mar 4;12:614946. doi: 10.3389/fphys.2021.614946. eCollection 2021. Front Physiol. 2021. PMID: 33746768 Free PMC article. Review.
-
Long-term changes in heart rate and electrical remodeling contribute to alternans formation in heart failure: a patient-specific in silico study.Am J Physiol Heart Circ Physiol. 2023 Aug 1;325(2):H414-H431. doi: 10.1152/ajpheart.00220.2023. Epub 2023 Jul 7. Am J Physiol Heart Circ Physiol. 2023. PMID: 37417871 Free PMC article.
References
-
- La Rovere M. T., Pinna G. D., Maestri R., Mortara A., Capomolla S., Febo O., Ferrari R., Franchini M., Gnemmi M., Opasich C. et al., “Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients,” Circulation 107, 565–570 (2003). 10.1161/01.CIR.0000047275.25795.17 - DOI - PubMed
-
- Nayyar S., Hasan M. A., Roberts-Thomson K. C., Sullivan T., and Baumert M., “Effect of loss of heart rate variability on T-wave heterogeneity and QT variability in heart failure patients: Implications in ventricular arrhythmogenesis,” Cardiovasc. Eng. Technol. 8, 219–228 (2017). 10.1007/s13239-017-0299-9 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

