An updated review on bluetongue virus: epidemiology, pathobiology, and advances in diagnosis and control with special reference to India
- PMID: 33003985
- PMCID: PMC7655031
- DOI: 10.1080/01652176.2020.1831708
An updated review on bluetongue virus: epidemiology, pathobiology, and advances in diagnosis and control with special reference to India
Abstract
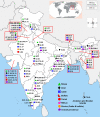
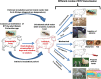
Bluetongue (BT) is an economically important, non-contagious viral disease of domestic and wild ruminants. BT is caused by BT virus (BTV) and it belongs to the genus Orbivirus and family Reoviridae. BTV is transmitted by Culicoides midges and causes clinical disease in sheep, white-tailed deer, pronghorn antelope, bighorn sheep, and subclinical manifestation in cattle, goats and camelids. BT is a World Organization for Animal Health (OIE) listed multispecies disease and causes great socio-economic losses. To date, 28 serotypes of BTV have been reported worldwide and 23 serotypes have been reported from India. Transplacental transmission (TPT) and fetal abnormalities in ruminants had been reported with cell culture adopted live-attenuated vaccine strains of BTV. However, emergence of BTV-8 in Europe during 2006, confirmed TPT of wild-type/field strains of BTV. Diagnosis of BT is more important for control of disease and to ensure BTV-free trade of animals and their products. Reverse transcription polymerase chain reaction, agar gel immunodiffusion assay and competitive enzyme-linked immunosorbent assay are found to be sensitive and OIE recommended tests for diagnosis of BTV for international trade. Control measures include mass vaccination (most effective method), serological and entomological surveillance, forming restriction zones and sentinel programs. Major hindrances with control of BT in India are the presence of multiple BTV serotypes, high density of ruminant and vector populations. A pentavalent inactivated, adjuvanted vaccine is administered currently in India to control BT. Recombinant vaccines with DIVA strategies are urgently needed to combat this disease. This review is the first to summarise the seroprevalence of BTV in India for 40 years, economic impact and pathobiology.
Keywords: Cattle; Indian scenario; bluetongue virus; control; diagnosis; epidemiology; goat; immune responses; mice model; pathogenesis; pathology; sheep; vaccination.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
-
- Afshar A. 1994. Bluetongue: laboratory diagnosis. Comp Immunol Microbiol Infect Dis. 17(3–4):221–242. - PubMed
-
- Afshar A, Dubuc C, Dulac GC, Thomas FC, Nielsen K, Henning D.. 1991. Dot immunoperoxidase assay using monoclonal antibody for detection of bluetongue virus antigens. J Virol Methods. 31(1):105–112. - PubMed
-
- Afshar A, Eaton BT, Wright PF, Pearson JE, Anderson J, Jeggo M, Trotter HC.. 1992. Competitive ELISA for serodiagnosis of bluetongue: evaluation of group-specific monoclonal antibodies and expressed VP7 antigen. J Vet Diagn Invest. 4(3):231–237. - PubMed
-
- Afshar A, Thomas FC, Wright PF, Shapiro JL, Anderson J.. 1989. Comparison of competitive ELISA, indirect ELISA and standard AGID tests for detecting bluetongue virus antibodies in cattle and sheep. Vet Rec. 124(6):136–141. - PubMed
-
- Akhtar S, Howe RR, Jadoon JK, Naqvi MA.. 1995. Prevalence of five serotypes of bluetongue virus in a Rambouillet sheep flock in Pakistan. Vet Rec. 136(19):495–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
