CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy
- PMID: 33004541
- PMCID: PMC7534680
- DOI: 10.1136/jitc-2020-000847
CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy
Abstract
Background: Combination treatment with chemotherapy and immune checkpoint inhibitors (ICIs) has demonstrated meaningful clinical benefit to patients. However, chemotherapy-induced damage to the immune system can potentially diminish the efficacy of chemotherapy/ICI combinations. Trilaciclib, a highly potent, selective and reversible cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor in development to preserve hematopoietic stem and progenitor cells and immune system function during chemotherapy, has demonstrated proof of concept in recent clinical trials. Furthermore, CDK4/6 inhibition has been shown to augment T-cell activation and antitumor immunity in preclinical settings. Therefore, addition of trilaciclib has the potential to further enhance the efficacy of chemotherapy and ICI combinations.
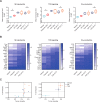
Methods: In murine syngeneic tumor models, a schedule of 3 weekly doses of trilaciclib was combined with chemotherapy/ICI regimens to assess the effect of transient CDK4/6 inhibition on antitumor response and intratumor T-cell proliferation and function. Peripheral T-cell status was also analyzed in patients with small cell lung cancer (SCLC) treated with chemotherapy with or without trilaciclib to gain insights into the effect of transient exposure of trilaciclib on T-cell activation.
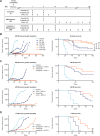
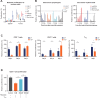
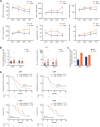
Results: Preclinically, the addition of trilaciclib to chemotherapy/ICI regimens enhanced antitumor response and overall survival compared with chemotherapy and ICI combinations alone. This effect is associated with the modulation of the proliferation and composition of T-cell subsets in the tumor microenvironment and increased effector function. Transient exposure of trilaciclib in patients with SCLC during chemotherapy treatment both preserved and increased peripheral lymphocyte counts and enhanced T-cell activation, suggesting that trilaciclib not only preserved but also enhanced immune system function.
Conclusions: Transient CDK4/6 inhibition by trilaciclib was sufficient to enhance and prolong the duration of the antitumor response by chemotherapy/ICI combinations, suggesting a role for the transient cell cycle arrest of tumor immune infiltrates in remodeling the tumor microenvironment. These results provide a rationale for combining trilaciclib with chemotherapy/ICI regimens to improve antitumor efficacy in patients with cancer.
Trial registration: ClinicalTrials.gov NCT02499770.
Keywords: clinical trials; drug evaluation; investigational; phase II as topic; preclinical; therapies.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AYL, PJR, ZY and JCS were employees of G1 Therapeutics at the time of the study. AYL is an employee of Locus Biosciences. PJR and JCS are employees of Arc Therapeutics. ZY is an employee of Everest Clinical Research. JAS and RKM are employees of G1 Therapeutics. JAR was an employee of Adaptive Biotechnologies at the time of the study. JH is an employee of and has a financial interest in Adaptive Biotechnologies. JMW has received honorarium from G1 Therapeutics. JMW, KHD and TKO received research funding from G1 Therapeutics (to their institution).
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
