Extracellular vesicle-associated VEGF-C promotes lymphangiogenesis and immune cells infiltration in endometriosis
- PMID: 33004630
- PMCID: PMC7568311
- DOI: 10.1073/pnas.1920037117
Extracellular vesicle-associated VEGF-C promotes lymphangiogenesis and immune cells infiltration in endometriosis
Abstract
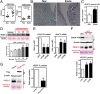
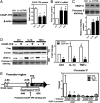
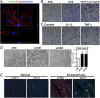
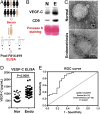
Endometriosis is a highly prevalent gynecological disease with severe negative impacts on life quality and financial burden. Unfortunately, there is no cure for this disease, which highlights the need for further investigation about the pathophysiology of this disease to provide clues for developing novel therapeutic regimens. Herein, we identified that vascular endothelial growth factor (VEGF)-C, a potent lymphangiogenic factor, is up-regulated in endometriotic cells and contributes to increased lymphangiogenesis. Bioinformatic analysis and molecular biological characterization revealed that VEGF-C is negatively regulated by an orphan nuclear receptor, chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII). Further studies demonstrated that proinflammatory cytokines, via suppression of COUP-TFII level, induce VEGF-C overexpression. More importantly, we show that functional VEGF-C is transported by extracellular vesicles (EVs) to enhance the lymphangiogenic ability of lymphatic endothelial cells. Autotransplanted mouse model of endometriosis showed lenvatinib treatment abrogated the increased lymphatic vessels development in the endometriotic lesion, enlarged retroperitoneal lymph nodes, and immune cells infiltration, indicating that blocking VEGF-C signaling can reduce local chronic inflammation and concomitantly endometriosis development. Evaluation of EV-transmitted VEGF-C from patients' sera demonstrates it is a reliable noninvasive way for clinical diagnosis. Taken together, we identify the vicious cycle of inflammation, COUP-TFII, VEGF-C, and lymphangiogenesis in the endometriotic microenvironment, which opens up new horizons in understanding the pathophysiology of endometriosis. VEGF-C not only can serve as a diagnostic biomarker but also a molecular target for developing therapeutic regimens.
Keywords: COUP-TFII; EV; VEGF-C; biomarker; lymphangiogenesis.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Sampson J. A., Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 14, 422–425 (1927). - PubMed
-
- Cramer D. W., Missmer S. A., The epidemiology of endometriosis. Ann. N. Y. Acad. Sci. 955, 11–22 (2002). - PubMed
-
- Halme J., Hammond M. G., Hulka J. F., Raj S. G., Talbert L. M., Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 64, 151–154 (1984). - PubMed
-
- Hsiao K. Y., Lin S. C., Wu M. H., Tsai S. J., Pathological functions of hypoxia in endometriosis. Front. Biosci. (Elite Ed.) 7, 309–321 (2015). - PubMed
-
- Wu M. H., Hsiao K. Y., Tsai S. J., Hypoxia: The force of endometriosis. J. Obstet. Gynaecol. Res. 45, 532–541 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

