Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau
- PMID: 33004675
- PMCID: PMC7818661
- DOI: 10.1126/science.aay8826
Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau
Abstract
Neurodegeneration in Alzheimer's disease (AD) is closely associated with the accumulation of pathologic tau aggregates in the form of neurofibrillary tangles. We found that a p.Asp395Gly mutation in VCP (valosin-containing protein) was associated with dementia characterized neuropathologically by neuronal vacuoles and neurofibrillary tangles. Moreover, VCP appeared to exhibit tau disaggregase activity in vitro, which was impaired by the p.Asp395Gly mutation. Additionally, intracerebral microinjection of pathologic tau led to increased tau aggregates in mice in which p.Asp395Gly VCP mice was knocked in, as compared with injected wild-type mice. These findings suggest that p.Asp395Gly VCP is an autosomal-dominant genetic mutation associated with neurofibrillary degeneration in part owing to reduced tau disaggregation, raising the possibility that VCP may represent a therapeutic target for the treatment of AD.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
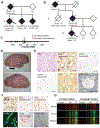



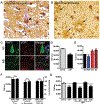


References
-
- Kovacs GG, Tauopathies. Handb Clin Neurol 145, 355 (2017). - PubMed
-
- Spillantini MG, Goedert M, Tau pathology and neurodegeneration. Lancet Neurol 12, 609 (June, 2013). - PubMed
-
- Lee VM, Goedert M, Trojanowski JQ, Neurodegenerative tauopathies. Annu Rev Neurosci 24, 1121 (2001). - PubMed
-
- van den Boom J, Meyer H, VCP/p97-Mediated Unfolding as a Principle in Protein Homeostasis and Signaling. Mol Cell 69, 182 (January 18, 2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

