CAR T cells targeting tumor endothelial marker CLEC14A inhibit tumor growth
- PMID: 33004686
- PMCID: PMC7566713
- DOI: 10.1172/jci.insight.138808
CAR T cells targeting tumor endothelial marker CLEC14A inhibit tumor growth
Abstract
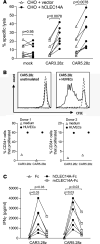
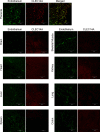
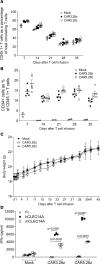
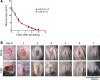
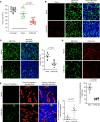
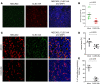
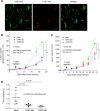
Engineering T cells to express chimeric antigen receptors (CARs) specific for antigens on hematological cancers has yielded remarkable clinical responses, but with solid tumors, benefit has been more limited. This may reflect lack of suitable target antigens, immune evasion mechanisms in malignant cells, and/or lack of T cell infiltration into tumors. An alternative approach, to circumvent these problems, is targeting the tumor vasculature rather than the malignant cells directly. CLEC14A is a glycoprotein selectively overexpressed on the vasculature of many solid human cancers and is, therefore, of considerable interest as a target antigen. Here, we generated CARs from 2 CLEC14A-specific antibodies and expressed them in T cells. In vitro studies demonstrated that, when exposed to their target antigen, these engineered T cells proliferate, release IFN-γ, and mediate cytotoxicity. Infusing CAR engineered T cells into healthy mice showed no signs of toxicity, yet these T cells targeted tumor tissue and significantly inhibited tumor growth in 3 mouse models of cancer (Rip-Tag2, mPDAC, and Lewis lung carcinoma). Reduced tumor burden also correlated with significant loss of CLEC14A expression and reduced vascular density within malignant tissues. These data suggest the tumor vasculature can be safely and effectively targeted with CLEC14A-specific CAR T cells, offering a potent and widely applicable therapy for cancer.
Keywords: Cancer immunotherapy; Immunology; Immunotherapy; Oncology; T cells.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

