Effects of hydrogen-rich water prepared by alternating-current-electrolysis on antioxidant activity, DNA oxidative injuries, and diabetes-related markers
- PMID: 33004708
- PMCID: PMC8086617
- DOI: 10.4103/2045-9912.296041
Effects of hydrogen-rich water prepared by alternating-current-electrolysis on antioxidant activity, DNA oxidative injuries, and diabetes-related markers
Abstract
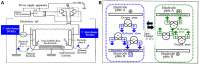
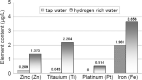
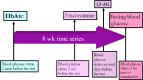
Hydrogen-rich water is conventionally prepared by direct current-electrolysis, but has been not or scarcely prepared by alternating current (AC)-electrolysis. The AC preparations from tap water for 20-30 minutes exhibit a dissolved hydrogen concentration of 1.55 mg/L, which was close to the theoretical maximum value of 1.6 mg/L. These preparations also displayed an oxidation-reduction potential of -270 mV (tap water: +576 mV) and pH of 7.7-7.8, being closer to physiological values of body fluids than general types of direct current-electrolytic hydrogen-rich water. We examined whether AC-electrolytic hydrogen-water is retained for hydrogen-abundance after boiling or for antioxidant abilities, and whether the oral administration of this water is clinically effective for diabetes and prevention against systemic DNA-oxidative injuries. 5,5-Dimethyl-1-pyrroline-N-oxide spin trapping and electron spin resonance revealed that the hydrogen-rich water generated by AC-electrolysis exhibited hydroxyl-radical-scavenging activities. Laser nanoparticle tracking method revealed that nanoparticle suspensions as abundant as 5.4 × 107/mL were efficiently retained (up to 3.5 × 107/mL) even after boiling for 10 minutes, being thermodynamically contrary to Henry's law. Oral intake of hydrogen-rich water, 1500 mL per day, lasted for 8 weeks in nine people with the diabetes-related serum markers beyond the normal ranges. The subjects exhibited significant tendencies for the decreased fasting blood glucose and fructosamine, and for the increased 1,5-anhydro-D-glucitol, concomitantly with significant decreases in urinary 8-hydroxy-2-deoxyguanosine contents and its rate of generation. Hydrogen-rich water prepared by AC-electrolysis may be effective in improving diverse diabetes-related markers and systemic DNA oxidative injuries through the formation of abundant heat-resistant nanobubbles and the increased hydrogen concentrations. The study protocol was officially approved by the Medical Ethics Committee of the Japanese Center for Anti-Aging Medical Sciences (approval No. 01S02) on September 15, 2009.
Keywords: DNA-oxidative injuries; alternating current-electrolysis; antioxidant activity; diabetes; hydrogen-rich water; reactive oxygen species.
Conflict of interest statement
None
Figures




References
-
- Kato S, Saitoh Y, Iwai K, Miwa N. Hydrogen-rich electrolyzed warm water represses wrinkle formation against UVA ray together with type-I collagen production and oxidative-stress diminishment in fibroblasts and cell-injury prevention in keratinocytes. J Photochem Photobiol B. 2012;106:24–33. - PubMed
-
- Kato S, Saitoh Y, Miwa N. Inhibitions by hydrogen-occluding silica microcluster to melanogenesis in human pigment cells and tyrosinase reaction. J Nanosci Nanotechnol. 2013;13:52–59. - PubMed
-
- Xiao L, Miwa N. Hydrogen-rich water achieves cytoprotection from oxidative stress injury in human gingival fibroblasts in culture or 3D-tissue equivalents, and wound-healing promotion, together with ROS-scavenging and relief from glutathione diminishment. Hum Cell. 2017;30:72–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

