Sulforaphane-cysteine inhibited migration and invasion via enhancing mitophagosome fusion to lysosome in human glioblastoma cells
- PMID: 33004792
- PMCID: PMC7530759
- DOI: 10.1038/s41419-020-03024-5
Sulforaphane-cysteine inhibited migration and invasion via enhancing mitophagosome fusion to lysosome in human glioblastoma cells
Abstract
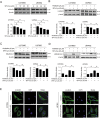
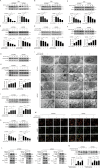
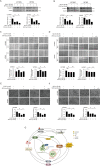
Here we uncovered the involved subcellular mechanisms that sulforaphane-cysteine (SFN-Cys) inhibited invasion in human glioblastoma (GBM). SFN-Cys significantly upregulated 45 and downregulated 14 microtubule-, mitophagy-, and invasion-associated proteins in GBM cells via HPLC-MS/MS and GEO ontology analysis; SFN-Cys disrupted microtubule by ERK1/2 phosphorylation-mediated downregulation of α-tubulin and Stathmin-1 leading to the inhibition of cell migration and invasion; SFN-Cys downregulated invasion-associated Claudin-5 and S100A4, and decreased the interaction of α-tubulin to Claudin-5. Knockdown of Claudin-5 and S100A4 significantly reduced the migration and invasion. Besides, SFN-Cys lowered the expressions of α-tubulin-mediated mitophagy-associated proteins Bnip3 and Nix. Transmission electron microscopy showed more membrane-deficient mitochondria and accumulated mitophagosomes in GBM cells, and mitochondria fusion might be downregulated because that SFN-Cys downregulated mitochondrial fusion protein OPA1. SFN-Cys increased the colocalization and interplay of LC3 to lysosomal membrane-associated protein LAMP1, aggravating the fusion of mitophagosome to lysosome. Nevertheless, SFN-Cys inhibited the lysosomal proteolytic capacity causing LC3II/LC3I elevation but autophagy substrate SQSTM1/p62 was not changed, mitophagosome accumulation, and the inhibition of migration and invasion in GBM cells. These results will help us develop high-efficiency and low-toxicity anticancer drugs to inhibit migration and invasion in GBM.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

