An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure
- PMID: 33004819
- PMCID: PMC7529912
- DOI: 10.1038/s41598-020-73055-7
An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure
Abstract
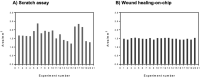
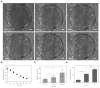
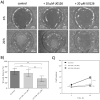
Dermal fibroblast cell migration is a key process in a physiological wound healing. Therefore, the analysis of cell migration is crucial for wound healing research. In this study, lab-on-a-chip technology was used to investigate the effects of basic fibroblast growth factor (bFGF), mitomycin C (MMC), MEK1/2 inhibitor (U0126) and fetal calf serum (FCS) on human dermal fibroblast cell migration. The microdevice was fabricated consisting of microchannels, pneumatic lines and pneumatically-activated actuators by xurographic rapid prototyping. In contrast to current approaches in in vitro wound healing such as scratch assays and silicone inserts in wellplate format, which show high variability and poor reproducibility, the current system aims to automate the wounding procedure at high precision and reproducibility using lab-on-a-chip. Traumatic wounding was simulated on-chip on fibroblast cell monolayers by applying air pressure on the flexible circular membrane actuator. Wound closure was monitored using light microscopy and cell migration was evaluated using image analysis. The pneumatically controlled system generates highly reproducible wound sizes compared to the conventional wound healing assay. As proof-of-principle study wound healing was investigated in the presence of several stimulatory and inhibitory substances and culture including bFGF, MMC, U0126 MEK1/2 inhibitor as well as serum starvation to demonstrate the broad applicability of the proposed miniaturized culture microsystem.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

