Radionecrosis and cellular changes in small volume stereotactic brain radiosurgery in a porcine model
- PMID: 33004849
- PMCID: PMC7529917
- DOI: 10.1038/s41598-020-72876-w
Radionecrosis and cellular changes in small volume stereotactic brain radiosurgery in a porcine model
Abstract
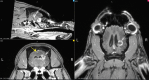
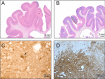
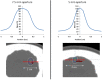
Stereotactic radiosurgery (SRS) has proven an effective tool for the treatment of brain tumors, arteriovenous malformation, and functional conditions. However, radiation-induced therapeutic effect in viable cells in functional SRS is also suggested. Evaluation of the proposed modulatory effect of irradiation on neuronal activity without causing cellular death requires the knowledge of radiation dose tolerance at very small tissue volume. Therefore, we aimed to establish a porcine model to study the effects of ultra-high radiosurgical doses in small volumes of the brain. Five minipigs received focal stereotactic radiosurgery with single large doses of 40-100 Gy to 5-7.5 mm fields in the left primary motor cortex and the right subcortical white matter, and one animal remained as unirradiated control. The animals were followed-up with serial MRI, PET scans, and histology 6 months post-radiation. We observed a dose-dependent relation of the histological and MRI changes at 6 months post-radiation. The necrotic lesions were seen in the grey matter at 100 Gy and in white matter at 60 Gy. Furthermore, small volume radiosurgery at different dose levels induced vascular, as well as neuronal cell changes and glial cell remodeling.
Conflict of interest statement
There are no potential conflicts of interest for Aarhus University Hospital and CENSE employees (Dariusz Orlowski, Andreas N. Glud, Hamed Zaer, Slávka Lukacova, Kim Hansen Vang, Morten Høyer, Morten Bjørn Jensen, Esben Schjødt Worm, Rune Hansen, Lone Hoffman and Jens C. H. Sørensen). Bret M. Schneider and John R. Adler are employed by Zap Surgical Systems Inc., which financed the study, own stock, and have patents in the field. ZAP surgical system besides financing the experiment had not influenced the conclusions of the results presented in this paper.
Figures




References
-
- Leksell L. Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir. Scand. 1971;137:311–314. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

