Meiotic analyses show adaptations to maintenance of fertility in X1Y1X2Y2X3Y3X4Y4X5Y5 system of amazon frog Leptodactylus pentadactylus (Laurenti, 1768)
- PMID: 33004883
- PMCID: PMC7529792
- DOI: 10.1038/s41598-020-72867-x
Meiotic analyses show adaptations to maintenance of fertility in X1Y1X2Y2X3Y3X4Y4X5Y5 system of amazon frog Leptodactylus pentadactylus (Laurenti, 1768)
Abstract
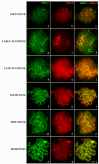
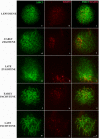
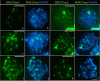
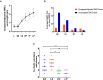
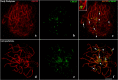
Heterozygous chromosomal rearrangements can result in failures during the meiotic cycle and the apoptosis of germline, making carrier individuals infertile. The Amazon frog Leptodactylus pentadactylus has a meiotic multivalent, composed of 12 sex chromosomes. The mechanisms by which this multi-chromosome system maintains fertility in males of this species remain undetermined. In this study we investigated the meiotic behavior of this multivalent to understand how synapse, recombination and epigenetic modifications contribute to maintaining fertility and chromosomal sexual determination in this species. Our sample had 2n = 22, with a ring formed by ten chromosomes in meiosis, indicating a new system of sex determination for this species (X1Y1X2Y2X3Y3X4Y4X5Y5). Synapsis occurs in the homologous terminal portion of the chromosomes, while part of the heterologous interstitial regions performed synaptic adjustment. The multivalent center remains asynaptic until the end of pachytene, with interlocks, gaps and rich-chromatin in histone H2A phosphorylation at serine 139 (γH2AX), suggesting transcriptional silence. In late pachytene, paired regions show repair of double strand-breaks (DSBs) with RAD51 homolog 1 (Rad51). These findings suggest that Rad51 persistence creates positive feedback at the pachytene checkpoint, allowing meiosis I to progress normally. Additionally, histone H3 trimethylation at lysine 27 in the pericentromeric heterochromatin of this anuran can suppress recombination in this region, preventing failed chromosomal segregation. Taken together, these results indicate that these meiotic adaptations are required for maintenance of fertility in L. pentadactylus.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

