Progressive optic atrophy in a retinal ganglion cell-specific mouse model of complex I deficiency
- PMID: 33004958
- PMCID: PMC7529752
- DOI: 10.1038/s41598-020-73353-0
Progressive optic atrophy in a retinal ganglion cell-specific mouse model of complex I deficiency
Abstract
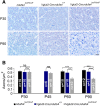
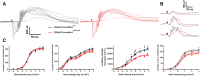
Optic atrophy resulting from retinal ganglion cell (RGC) degeneration is a prominent ocular manifestation of mitochondrial dysfunction. Although transgenic mice lacking the mitochondrial complex I accessory subunit NDUFS4 develop early-onset optic atrophy, severe systemic mitochondrial dysfunction leads to very early death and makes this mouse line impractical for studying the pathobiology of mitochondrial optic neuropathies. Theoretically, RGC-specific inactivation of ndufs4 would allow characterization of RGC degeneration over a longer time course, provided that RGC death from mitochondrial dysfunction is a cell-autonomous process. We demonstrate that the vesicular glutamate transporter VGLUT2 may be exploited to drive robust Cre recombinase expression in RGCs without any expression observed in directly neighboring retinal cell types. Deletion of ndufs4 in RGCs resulted in reduced expression of NDUFS4 protein within the optic nerves of Vglut2-Cre;ndufs4loxP/loxP mice. RGC degeneration in Vglut2-Cre;ndufs4loxP/loxP retinas commenced around postnatal day 45 (P45) and progressed to loss of two-thirds of RGCs by P90, confirming that intrinsic complex I dysfunction is sufficient to induce RGC death. The rapidly-developing optic atrophy makes the Vglut2-Cre;ndufs4loxP/loxP mouse line a promising preclinical model for testing therapies for currently untreatable mitochondrial optic neuropathies such as Leber Hereditary Optic Neuropathy.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

