A generalized workflow for conducting electric field-optimized, fMRI-guided, transcranial magnetic stimulation
- PMID: 33005039
- PMCID: PMC8123368
- DOI: 10.1038/s41596-020-0387-4
A generalized workflow for conducting electric field-optimized, fMRI-guided, transcranial magnetic stimulation
Abstract
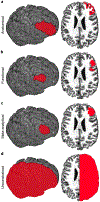
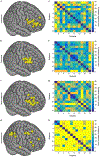
Transcranial magnetic stimulation (TMS) is a noninvasive method to stimulate the cerebral cortex that has applications in psychiatry, such as in the treatment of depression and anxiety. Although many TMS targeting methods that use figure-8 coils exist, many do not account for individual differences in anatomy or are not generalizable across target sites. This protocol combines functional magnetic resonance imaging (fMRI) and iterative electric-field (E-field) modeling in a generalized approach to subject-specific TMS targeting that is capable of optimizing the stimulation site and TMS coil orientation. To apply this protocol, the user should (i) operationally define a region of interest (ROI), (ii) generate the head model from the structural MRI data, (iii) preprocess the functional MRI data, (iv) identify the single-subject stimulation site within the ROI, and (iv) conduct E-field modeling to identify the optimal coil orientation. In comparison with standard targeting methods, this approach demonstrates (i) reduced variability in the stimulation site across subjects, (ii) reduced scalp-to-cortical-target distance, and (iii) reduced variability in optimal coil orientation. Execution of this protocol requires intermediate-level skills in structural and functional MRI processing. This protocol takes ~24 h to complete and demonstrates how constrained fMRI targeting combined with iterative E-field modeling can be used as a general method to optimize both the TMS coil site and its orientation.
Trial registration: ClinicalTrials.gov NCT03027414.
Figures





References
-
- Laakso I, Murakami T, Hirata A & Ugawa Y Where and what TMS activates: experiments and modeling. Brain Stimul. 11, 166–174 (2017). - PubMed
-
- Terao Y & Ugawa Y Basic mechanisms of TMS. J. Clin. Neurophysiol 19, 322–343 (2002). - PubMed
-
- Thielscher A & Kammer T Electric field properties of two commercial figure-8 coils in TMS: calculation of focality and efficiency. Clin. Neurophysiol 115, 1697–1708 (2004). - PubMed
-
- Roth Y, Amir A, Levkovitz Y & Zangen A Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J. Clin. Neurophysiol 24, 31–38 (2007). - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

