Impact of Ovarian Aging in Reproduction: From Telomeres and Mice Models to Ovarian Rejuvenation
- PMID: 33005120
- PMCID: PMC7513441
Impact of Ovarian Aging in Reproduction: From Telomeres and Mice Models to Ovarian Rejuvenation
Abstract
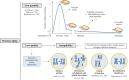
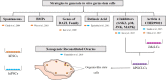
The trend in our society to delay procreation increases the difficulty to conceive spontaneously. Thus, there is a growing need to use assisted reproduction technologies (ART) to form a family. With advanced maternal age, ovaries not only produce a lower number of oocytes after ovarian stimulation but also a lower quality-mainly aneuploidies-requiring further complex analysis to avoid complications during implantation and pregnancy. Although there are different options to have a child at advanced maternal age (like donor eggs), this is not the preferred choice for most patients. Unless women had cryopreserved their eggs at a younger age, reproductive medicine should try to optimize their opportunities to become pregnant with their own oocytes, when chances of success are reasonable. Aging has many causes, but telomere attrition is ultimately one of the main pathways involved in this process. Several reports link telomere biology and reproduction, but the molecular reasons for the rapid loss of ovarian function at middle age are still elusive. This review will focus on the knowledge acquired during the last years about ovarian aging and disease, both in mouse models of reproductive senescence and in humans with ovarian failure, and the implication of telomeres in this process. In addition, the review will discuss recent results on ovarian rejuvenation, achieved with stem cell therapies that are currently under study, or ovarian reactivation by tissue fragmentation and the attempts to generate oocytes in vitro.
Keywords: Telomeres; iPS cells; ovary; reproduction; stem cells.
Copyright ©2020, Yale Journal of Biology and Medicine.
Figures

Similar articles
-
Telomeres, aging and reproduction.Curr Opin Obstet Gynecol. 2022 Jun 1;34(3):151-158. doi: 10.1097/GCO.0000000000000779. Epub 2022 Mar 4. Curr Opin Obstet Gynecol. 2022. PMID: 35645014 Review.
-
Telomeres, Reproductive Aging, and Genomic Instability During Early Development.Reprod Sci. 2016 Dec;23(12):1612-1615. doi: 10.1177/1933719116676397. Reprod Sci. 2016. PMID: 27821557 Review.
-
Resveratrol protects against age-associated infertility in mice.Hum Reprod. 2013 Mar;28(3):707-17. doi: 10.1093/humrep/des437. Epub 2013 Jan 4. Hum Reprod. 2013. PMID: 23293221
-
Fertility, IVF and reproductive genetics.Curr Opin Obstet Gynecol. 2018 Jun;30(3):203-208. doi: 10.1097/GCO.0000000000000456. Curr Opin Obstet Gynecol. 2018. PMID: 29708900 Review.
-
Reproductive choices and outcomes after freezing oocytes for medical reasons: a follow-up study.Hum Reprod. 2014 Sep;29(9):1925-30. doi: 10.1093/humrep/deu137. Epub 2014 Jun 20. Hum Reprod. 2014. PMID: 24951490
Cited by
-
Idiopathic early ovarian aging: is there a relation with premenopausal accelerated biological aging in young women with diminished response to ART?J Assist Reprod Genet. 2021 Nov;38(11):3027-3038. doi: 10.1007/s10815-021-02326-7. Epub 2021 Oct 1. J Assist Reprod Genet. 2021. PMID: 34599460 Free PMC article.
-
Electrical Stimulation Rejuvenates Tunicates: Altered Stem Cell and Immune Activity.bioRxiv [Preprint]. 2025 May 21:2025.05.17.654683. doi: 10.1101/2025.05.17.654683. bioRxiv. 2025. PMID: 40475539 Free PMC article. Preprint.
-
Oxidative Stress Induces Telomere Dysfunction and Shortening in Human Oocytes of Advanced Age Donors.Cells. 2021 Jul 23;10(8):1866. doi: 10.3390/cells10081866. Cells. 2021. PMID: 34440635 Free PMC article.
-
Ovarian Telomerase and Female Fertility.Biomedicines. 2021 Jul 20;9(7):842. doi: 10.3390/biomedicines9070842. Biomedicines. 2021. PMID: 34356906 Free PMC article. Review.
-
Human ovarian aging is characterized by oxidative damage and mitochondrial dysfunction.Hum Reprod. 2023 Nov 2;38(11):2208-2220. doi: 10.1093/humrep/dead177. Hum Reprod. 2023. PMID: 37671592 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
