Ultrastructural Sperm Flagellum Defects in a Patient With CCDC39 Compound Heterozygous Mutations and Primary Ciliary Dyskinesia/ Situs Viscerum Inversus
- PMID: 33005176
- PMCID: PMC7483550
- DOI: 10.3389/fgene.2020.00974
Ultrastructural Sperm Flagellum Defects in a Patient With CCDC39 Compound Heterozygous Mutations and Primary Ciliary Dyskinesia/ Situs Viscerum Inversus
Abstract
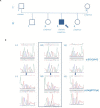
Introduction: Primary ciliary dyskinesia (PCD) is a rare autosomal recessive disease characterized by structural or functional motile cilia abnormalities. Up to 40 different genes seem, at the moment, to be involved in the pathogenesis of PCD. A number of ultrastructural defects have also been reported in sperm flagella, but the sperm mitochondrial membrane potential (MMP) has never been described in these cases. Aim: The aim of this study was to report the sperm MMP and ultrastructural abnormalities of the sperm flagella found in a patient with PCD and situs inversus (Kartagener syndrome) and its characterization from the genetic point of view. Methods: Transmission electronic microscopy (TEM) analysis was used to evaluate flagella ultrastructure. The genetic testing was performed by next-generation sequencing. Sperm DNA fragmentation and MMP were also evaluated by flow cytometry. Results: We report here the case of an 18-year-old male patient with PCD and situs inversus and severe oligo-astheno-teratozoospermia. TEM analysis of his spermatozoa showed an abnormal connecting piece. The mid piece appeared abnormally thickened, with cytoplasmic residue, dysplasia of fibrous sheath, loss of the outer dynein arms (ODAs), truncated inner dynein arms, and supernumerary outer fibers. The percentage of spermatozoa with fragmented DNA was normal, whereas a high percentage of spermatozoa had low MMP, suggesting an altered mitochondrial function. The genetic analysis showed the presence of c.610-2A > G, p.Arg811Cys compound heterozygous mutations in the CCDC39 gene. Conclusion: The case herein reported suggests that the high percentage of sperm with low MMP may play a role in the pathogenesis of asthenozoospermia in patients with Kartagener syndrome. In addition, we report, for the first time, the missense variant p.Arg811Cys in the CCDC39 gene in a patient with Kartagener syndrome. Although in silico analysis predicts its damaging potential, its clinical meaning remains unclear.
Keywords: CCDC151; CCDC39; Kartagener syndrome; asthenozoospermia; primary ciliary dyskinesia; situs inversus.
Copyright © 2020 Cannarella, Maniscalchi, Condorelli, Scalia, Guerri, La Vignera, Bertelli and Calogero.
Figures





References
LinkOut - more resources
Full Text Sources

