Adaptation and constraint shape the evolution of growth patterns in passerine birds across the globe
- PMID: 33005206
- PMCID: PMC7526225
- DOI: 10.1186/s12983-020-00377-7
Adaptation and constraint shape the evolution of growth patterns in passerine birds across the globe
Abstract
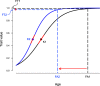
Background: Growth trajectories should be adapted to selective factors of each species' environment. However, major shaping forces of growth and development are unclear, especially when studying several traits at once. Birds provide an ideal opportunity to analyze growth patterns across species due to there being enough available data. We tested the relative importance of nest predation risk, the number of care-givers, nest height, foraging substrate, clutch size, and latitude on growth patterns of passerine birds (Passeriformes) using phylogenetic comparative methods. Specifically, we studied the evolution of fledging time, average and peak growth rates, and relative development at fledging of body mass and tarsus, wing, and tail length.
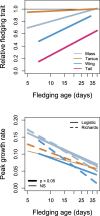
Results: Using a comprehensive literature search and data quality control, we obtained data on growth in 231 species based on 295 populations. Species with long development in the nest grew slowly and had well-developed traits at fledging. Species breeding under high nest predation risk, building their nests close to the ground, and those living in northern temperate regions fledged early and grew fast, sometimes fledging with less developed body mass and traits critical for locomotion (tarsus, wing, and tail). On the other hand, the number of caring adults, clutch size, and species' foraging substrate had very limited predictive value for growth patterns across passerine species.
Conclusions: Shortening of the nestling period was a primary means of accelerating development (in relation to nest predation, nest height, and latitude), sometimes supplemented by higher peak growth rates of body mass, tarsus, and wing (especially in relation to latitude). Overall growth patterns of passerines were adaptively tuned to nest predation risk and nest height, with northern temperate species having especially short nestling periods and fast growth rates of body mass, tarsus, and wing.
Keywords: Birds; Development; Growth rate; Latitude; Life history; Nest predation.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


References
-
- Gilbert SF, Epel D. Ecological Developmental Biology. Sunderland: Sinauer Associates; 2008.
-
- Wainwright PC, Reilly SM. Ecological morphology: integrative organismal biology. Chicago: University of Chicago Press; 1994.
-
- Norberg UM. How a long tail and changes in mass and wing shape affect the cost for flight in animals. Funct Ecol. 1995;9:48–54. doi: 10.2307/2390089. - DOI
-
- Zeffer A, Johansson LC, Marmebro Å. Functional correlation between habitat use and leg morphology in birds (Aves) Biol J Linn Soc. 2003;79:461–484. doi: 10.1046/j.1095-8312.2003.00200.x. - DOI
LinkOut - more resources
Full Text Sources

