Adaptive genetic potential and plasticity of trait variation in the foundation prairie grass Andropogon gerardii across the US Great Plains' climate gradient: Implications for climate change and restoration
- PMID: 33005227
- PMCID: PMC7513703
- DOI: 10.1111/eva.13028
Adaptive genetic potential and plasticity of trait variation in the foundation prairie grass Andropogon gerardii across the US Great Plains' climate gradient: Implications for climate change and restoration
Abstract
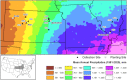
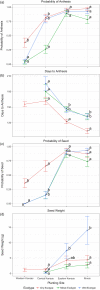
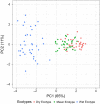
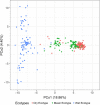
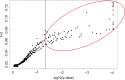
Plant response to climate depends on a species' adaptive potential. To address this, we used reciprocal gardens to detect genetic and environmental plasticity effects on phenotypic variation and combined with genetic analyses. Four reciprocal garden sites were planted with three regional ecotypes of Andropogon gerardii, a dominant Great Plains prairie grass, using dry, mesic, and wet ecotypes originating from western KS to Illinois that span 500-1,200 mm rainfall/year. We aimed to answer: (a) What is the relative role of genetic constraints and phenotypic plasticity in controlling phenotypes? (b) When planted in the homesite, is there a trait syndrome for each ecotype? (c) How are genotypes and phenotypes structured by climate? and (d) What are implications of these results for response to climate change and use of ecotypes for restoration? Surprisingly, we did not detect consistent local adaptation. Rather, we detected co-gradient variation primarily for most vegetative responses. All ecotypes were stunted in western KS. Eastward, the wet ecotype was increasingly robust relative to other ecotypes. In contrast, fitness showed evidence for local adaptation in wet and dry ecotypes with wet and mesic ecotypes producing little seed in western KS. Earlier flowering time in the dry ecotype suggests adaptation to end of season drought. Considering ecotype traits in homesite, the dry ecotype was characterized by reduced canopy area and diameter, short plants, and low vegetative biomass and putatively adapted to water limitation. The wet ecotype was robust, tall with high biomass, and wide leaves putatively adapted for the highly competitive, light-limited Eastern Great Plains. Ecotype differentiation was supported by random forest classification and PCA. We detected genetic differentiation and outlier genes associated with primarily precipitation. We identified candidate gene GA1 for which allele frequency associated with plant height. Sourcing of climate adapted ecotypes should be considered for restoration.
Keywords: Great Plains grasslands; drought; ecotypic variation; genetic differentiation; genome–environment interaction; local adaptation; phenotypic variation; precipitation; reciprocal gardens.
© 2020 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures




References
-
- Aitken, S. N. , & Whitlock, M. C. (2013). Assisted gene flow to facilitate local adaptation to climate change. Annual Review of Ecology, Evolution, and Systematics, 44, 367–388. 10.1146/annurev-ecolsys-110512-135747 - DOI
-
- Altman, N. , & Krzywinski, M. (2017). Points of Significance: Ensemble methods: bagging and random forests. Nature Methods, 14(10), 933–934. 10.1038/nmeth.4438 - DOI
-
- Anderson, J. T. , Eckhart, V. M. , & Geber, M. A. (2015). Experimental studies of adaptation in Clarkia xantiana. III. Phenotypic selection across a subspecies border. Evolution, 69(9), 2249–2261. - PubMed
-
- Anderson, J. T. , & Gezon, Z. J. (2015). Plasticity in functional traits in the context of climate change: A case study of the subalpine forb Boechera stricta (Brassicaceae). Global Change Biology, 21(4), 1689–1703. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

