The safety of upper gastrointestinal endoscopic biopsy in patients receiving antithrombic drugs. A single-centre prospective observational study
- PMID: 33005269
- PMCID: PMC7509902
- DOI: 10.5114/pg.2019.88622
The safety of upper gastrointestinal endoscopic biopsy in patients receiving antithrombic drugs. A single-centre prospective observational study
Abstract
Introduction: In July 2012, the Japan Gastroenterological Endoscopy Society updated their guidelines on gastroenterological endoscopy in patients undergoing antithrombotic therapy, although the safety of endoscopic procedures in patients receiving antithrombotic drugs has yet to be sufficiently studied.
Aim: This study evaluates the safety of upper gastroenterological endoscopic biopsy in patients receiving antithrombotic drugs. We evaluated the prospective observational safety of endoscopic biopsy performed in the endoscopy unit of our patients using antithrombotic drugs.
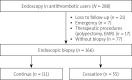
Material and methods: Oesophagogastroduodenoscopies (OGD) and biopsies performed at a single endoscopy unit between July 2018 and February 2019 were examined in this prospective observational study. Patients receiving antithrombotic drugs due to cardiovascular and neurological reasons, who underwent an endoscopic mucosal biopsy for diagnostic purposes, were included in the study.
Results: The study was completed with 166 patients who underwent an endoscopic biopsy, from whom a total of 327 biopsies taken. The patients were examined in two groups: those "receiving antithrombotic drugs" and those who had "stopped taking antithrombotic drugs". There was no statistically significant difference between the two groups with respect to bleeding.
Conclusions: This prospective observational study showed that performing an endoscopic biopsy without the cessation of antithrombotic drugs does not increase bleeding risk. Low-risk procedures, such as endoscopic mucosal biopsies, can be performed confidently by experienced endoscopists.
Keywords: antithrombotic; bleeding; endoscopic biopsy.
Copyright © 2020 Termedia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yasue H, Ogawa H, Tanaka H, et al. Effects of aspirin and trapidil on cardiovascular events after acute myocardial infarction. Japanese Antiplatelets Myocardial Infarction Study (JAMIS) Investigators. Am J Cardiol 1999; 83: 1308-13. - PubMed
-
- De Caterina R, Husted S, Wallentin L, et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC Working Group on Thrombosis-Task Force on Anticoagulants in Heart Disease position paper. J Am Coll Cardiol 2012; 59: 1413-25. - PubMed
-
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345: 494-502. - PubMed
-
- Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367: 1979-87. - PubMed
LinkOut - more resources
Full Text Sources