Molecular determinants of response to 5-fluorouracil-based chemotherapy in colorectal cancer: The undisputable role of micro-ribonucleic acids
- PMID: 33005290
- PMCID: PMC7510001
- DOI: 10.4251/wjgo.v12.i9.942
Molecular determinants of response to 5-fluorouracil-based chemotherapy in colorectal cancer: The undisputable role of micro-ribonucleic acids
Abstract
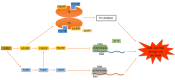
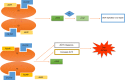
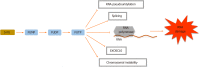
5-flurouracil (5-FU)-based chemotherapy is the main pharmacological therapy for advanced colorectal cancer (CRC). Despite significant progress in the treatment of CRC during the last decades, 5-FU drug resistance remains the most important cause of failure in CRC therapy. Resistance to 5-FU is a complex and multistep process. Different mechanisms including microsatellite instability, increased expression level of key enzyme thymidylate synthase and its polymorphism, increased level of 5-FU-activating enzymes and mutation of TP53 are proposed as the main determinants of resistance to 5-FU in CRC cells. Recently, micro-ribonucleic acids (miRNA) and their alterations were found to have a crucial role in 5-FU resistance. In this regard, the miRNA-mediated mechanisms of 5-FU drug resistance reside among the new fields of pharmacogenetics of CRC drug response that has not been completely discovered. Identification of the biological markers that are related to response to 5-FU-based chemotherapy is an emerging field of precision medicine. This approach will have an important role in defining those patients who are most likely to benefit from 5-FU-based chemotherapy in the future. Thereby, the identification of 5-FU drug resistance mechanisms is an essential step to predict and eventually overcome resistance. In the present comprehensive review, we will summarize the latest knowledge regarding the molecular determinants of response to 5-FU-based chemotherapy in CRC by emphasizing the role of miRNAs.
Keywords: 5-flurouracil; Chemotherapy resistance, Thymidylate synthase; Colorectal cancer; Micro-ribonucleic acid; Microsatellite instability; TP53.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no potential financial interests.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Malet-Martino M, Jolimaitre P, Martino R. The prodrugs of 5-fluorouracil. Curr Med Chem Anticancer Agents. 2002;2:267–310. - PubMed
-
- Eftekhar E, Naghibalhossaini F. Carcinoembryonic antigen expression level as a predictive factor for response to 5-fluorouracil in colorectal cancer. Mol Biol Rep. 2014;41:459–466. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

