Genome-wide noninvasive prenatal diagnosis of monogenic disorders: Current and future trends
- PMID: 33005308
- PMCID: PMC7509788
- DOI: 10.1016/j.csbj.2020.09.003
Genome-wide noninvasive prenatal diagnosis of monogenic disorders: Current and future trends
Abstract
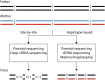
Noninvasive prenatal diagnosis (NIPD) is a risk-free alternative to invasive methods for prenatal diagnosis, e.g. amniocentesis. NIPD is based on the presence of fetal DNA within the mother's plasma cell-free DNA (cfDNA). Though currently available for various monogenic diseases through detection of point mutations, NIPD is limited to detecting one mutation or up to several genes simultaneously. Noninvasive prenatal whole exome/genome sequencing (WES/WGS) has demonstrated genome-wide detection of fetal point mutations in a few studies. However, Genome-wide NIPD of monogenic disorders currently has several challenges and limitations, mainly due to the small amounts of cfDNA and fetal-derived fragments, and the deep coverage required. Several approaches have been suggested for addressing these hurdles, based on various technologies and algorithms. The first relevant software tool, Hoobari, recently became available. Here we review the approaches proposed and the paths required to make genome-wide monogenic NIPD widely available in the clinic.
Keywords: Genome-wide; Monogenic disorders; NIPD; NIPT; Noninvasive prenatal diagnosis.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The Shomron Laboratory is supported by the Israel Science Foundation (ISF; 1852/16); Israeli Ministry of Defense, Office of Assistant Minister of Defense for Chemical, Biological, Radiological and Nuclear (CBRN) Defense; Foundation Fighting Blindness; The Edmond J. Safra Center for Bioinformatics at Tel Aviv University; Zimin Institute for Engineering Solutions Advancing Better Lives; Eric and Wendy Schmidt Breakthrough Innovative Research Award; Tel Aviv University Richard Eimert Research Fund on Solid Tumors; Djerassi-Elias Institute of Oncology; Canada-Montreal Friends of Tel Aviv University; Harold H. Marcus; Amy Friedkin; Natalio Garber; Kirschman Dvora Eleonora Fund for Parkinson's Disease; Joint funding between Tel Aviv University and Yonsei University; Israeli Ministry of Science and Technology, Israeli–Russia joint funding; Aufzien Family Center for the Prevention and Treatment of Parkinson’s Disease; and a generous donation from the Adelis Foundation.
Figures



References
-
- Agarwal K., Alfirevic Z. Pregnancy loss after chorionic villus sampling and genetic amniocentesis in twin pregnancies: a systematic review. Ultrasound Obstet Gynecol. 2012;40(2):128–134. - PubMed
-
- Akolekar R., Beta J., Picciarelli G., Ogilvie C., D’Antonio F. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45(1):16–26. - PubMed
-
- Hill M., Finning K., Martin P., Hogg J., Meaney C., Norbury G. Non-invasive prenatal determination of fetal sex: translating research into clinical practice. Clin Genet. 2011;80(1):68–75. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
