Context-dependent venom deployment and protein composition in two assassin bugs
- PMID: 33005355
- PMCID: PMC7520181
- DOI: 10.1002/ece3.6652
Context-dependent venom deployment and protein composition in two assassin bugs
Abstract
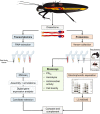
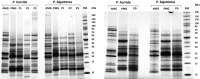
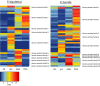
The Heteroptera are a diverse suborder of phytophagous, hematophagous, and zoophagous insects. The shift to zoophagy can be traced back to the transformation of salivary glands into venom glands, but the venom is used not only to kill and digest invertebrate prey but also as a defense strategy, mainly against vertebrates. In this study, we used an integrated transcriptomics and proteomics approach to compare the composition of venoms from the anterior main gland (AMG) and posterior main gland (PMG) of the reduviid bugs Platymeris biguttatus L. and Psytalla horrida Stål. In both species, the AMG and PMG secreted distinct protein mixtures with few interspecific differences. PMG venom consisted mostly of S1 proteases, redulysins, Ptu1-like peptides, and uncharacterized proteins, whereas AMG venom contained hemolysins and cystatins. There was a remarkable difference in biological activity between the AMG and PMG venoms, with only PMG venom conferring digestive, neurotoxic, hemolytic, antibacterial, and cytotoxic effects. Proteomic analysis of venom samples revealed the context-dependent use of AMG and PMG venom. Although both species secreted PMG venom alone to overwhelm their prey and facilitate digestion, the deployment of defensive venom was species-dependent. P. biguttatus almost exclusively used PMG venom for defense, whereas P. horrida secreted PMG venom in response to mild harassment but AMG venom in response to more intense harassment. This intriguing context-dependent use of defensive venom indicates that future research should focus on species-dependent differences in venom composition and defense strategies among predatory Heteroptera.
Keywords: assassin bug; defense venom; prey‐killing venom; proteomics; transcriptomics; zoophagy.
© 2020 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures





References
-
- Amino, R. , Martins, R. M. , Procopio, J. , Hirata, I. Y. , Juliano, M. A. , & Schenkman, S. (2002). Trialysin, a novel pore‐forming protein from saliva of hematophagous insects activated by limited proteolysis. Journal of Biological Chemistry, 277(8), 6207–6213. 10.1074/jbc.M109874200 - DOI - PubMed
-
- Amino, R. , Tanaka, A. S. , & Schenkman, S. (2001). Triapsin, an unusual activatable serine protease from the saliva of the hematophagous vector of Chagas' disease Triatoma infestans (Hemiptera: Reduviidae). Insect Biochemistry and Molecular Biology, 31(4–5), 465–472. 10.1016/S0965-1748(00)00151-X - DOI - PubMed
-
- Ayyachamy, V. K. , Sahayaraj, K. , & Rivers, D. B. (2016). Anti‐aggregation and Cytolytic Behaviour of Venomous Saliva of Rhynocoris fuscipes (Fab.)(Hemiptera: Reduviidae) in Response to Its Prey Hemocytes. Journal of the Entomological Research Society, 18(3), 1–13.
-
- Azevedo, D. D. O. , Zanuncio, J. C. , Zanuncio, J. S., Jr. , Martins, G. F. , Marques‐Silva, S. , Sossai, M. F. , & Serrão, J. E. (2007). Biochemical and morphological aspects of salivary glands of the predator Brontocoris tabidus (Heteroptera: Pentatomidae). Brazilian Archives of Biology and Technology, 50(3), 469–477. 10.1590/S1516-89132007000300013 - DOI
-
- Baptist, B. (1941). The morphology and physiology of the salivary glands of Hemiptera‐Heteroptera. Journal of Cell Science, 2(329), 91–139.
LinkOut - more resources
Full Text Sources

