Exploring Graphene and MoS2 Chips Based Surface Plasmon Resonance Biosensors for Diagnostic Applications
- PMID: 33005604
- PMCID: PMC7479841
- DOI: 10.3389/fchem.2020.00728
Exploring Graphene and MoS2 Chips Based Surface Plasmon Resonance Biosensors for Diagnostic Applications
Abstract
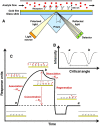
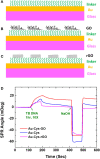
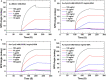
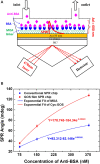
Until now, two-dimensional (2D) nanomaterials have been widely studied and applied in the biosensor field. Some of the advantages offered by these 2D materials include large specific surface area, high conductivity, and easy surface modification. This review discusses the use of 2D material in surface plasmon resonance (SPR) biosensor for diagnostic applications. Two-dimensional material reviewed includes graphene and molybdenum disulfide (MoS2). The discussion begins with a brief introduction to the general principles of the SPR biosensor. The discussion continues by explaining the properties and characteristics of each material and its effect on the performance of the SPR biosensor, in particular its sensitivity. This review concludes with some recent applications of graphene- and MoS2-based SPR biosensor in diagnostic applications.
Keywords: 2D materials; MoS2; biosensor; diagnostic; graphene; surface plasmon resonance.
Copyright © 2020 Nurrohman, Wang and Chiu.
Figures








References
-
- Agarwal S., Giri P., Prajapati Y. K., Chakrabarti P. (2016a). Effect of surface roughness on the performance of optical spr sensor for sucrose detection: fabrication, characterization, and simulation study. IEEE Sens. J. 16, 8865–8873. 10.1109/JSEN.2016.2615110 - DOI
-
- Agarwal S., Prajapati Y. K., Maurya J. B. (2016b). Effect of metallic adhesion layer thickness on surface roughness for sensing application. IEEE Photonics Technol. Lett. 28, 2415–2418. 10.1109/LPT.2016.2597856 - DOI
-
- Amieva J. C., Barroso J. L., Hernandez A. L. M., Santos C. V. (2016). “Graphene-based materials functionalization with natural polymeric biomolecules,” in Recent Advances in Graphene Research, ed Pramoda Kumar N. (Rijeka: InTech; ), 257–298.
Publication types
LinkOut - more resources
Full Text Sources

